Understanding chemical bonds in compounds and molecular structures is fundamental to chemistry. This comprehensive guide covers bond formation, Lewis structures, and molecular geometry.
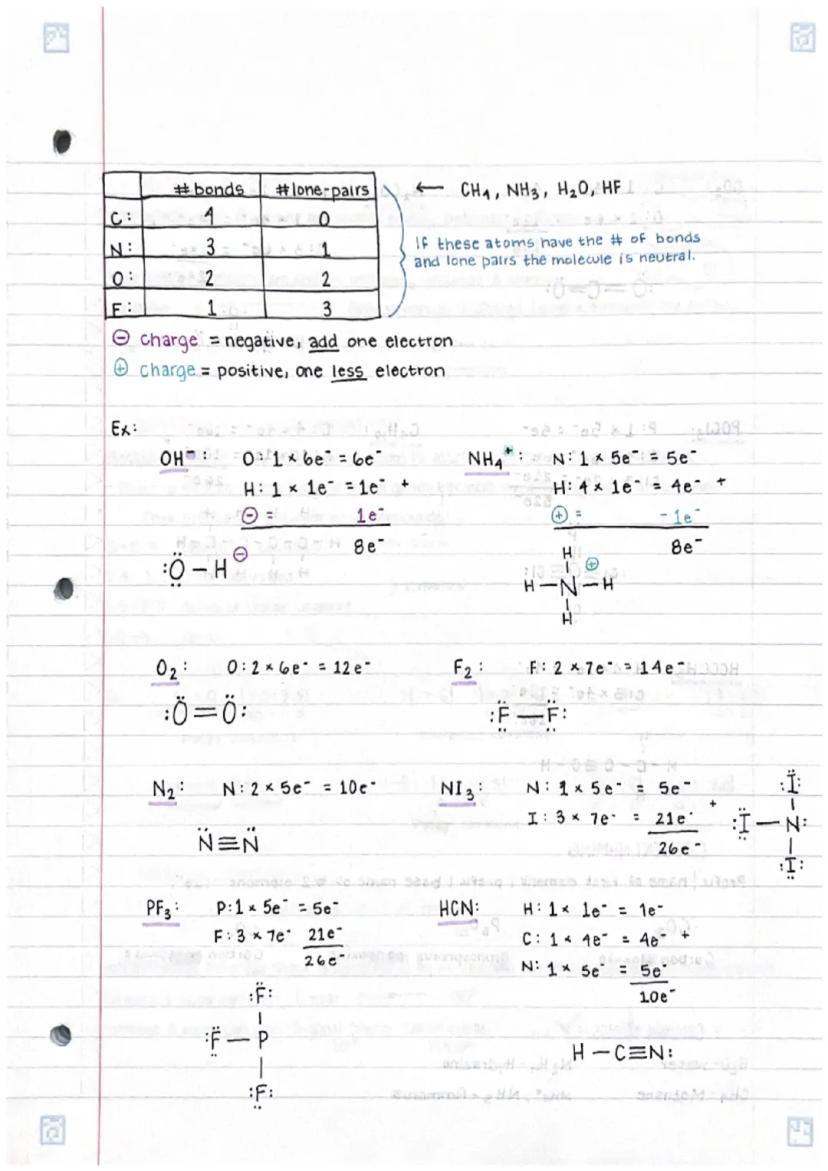
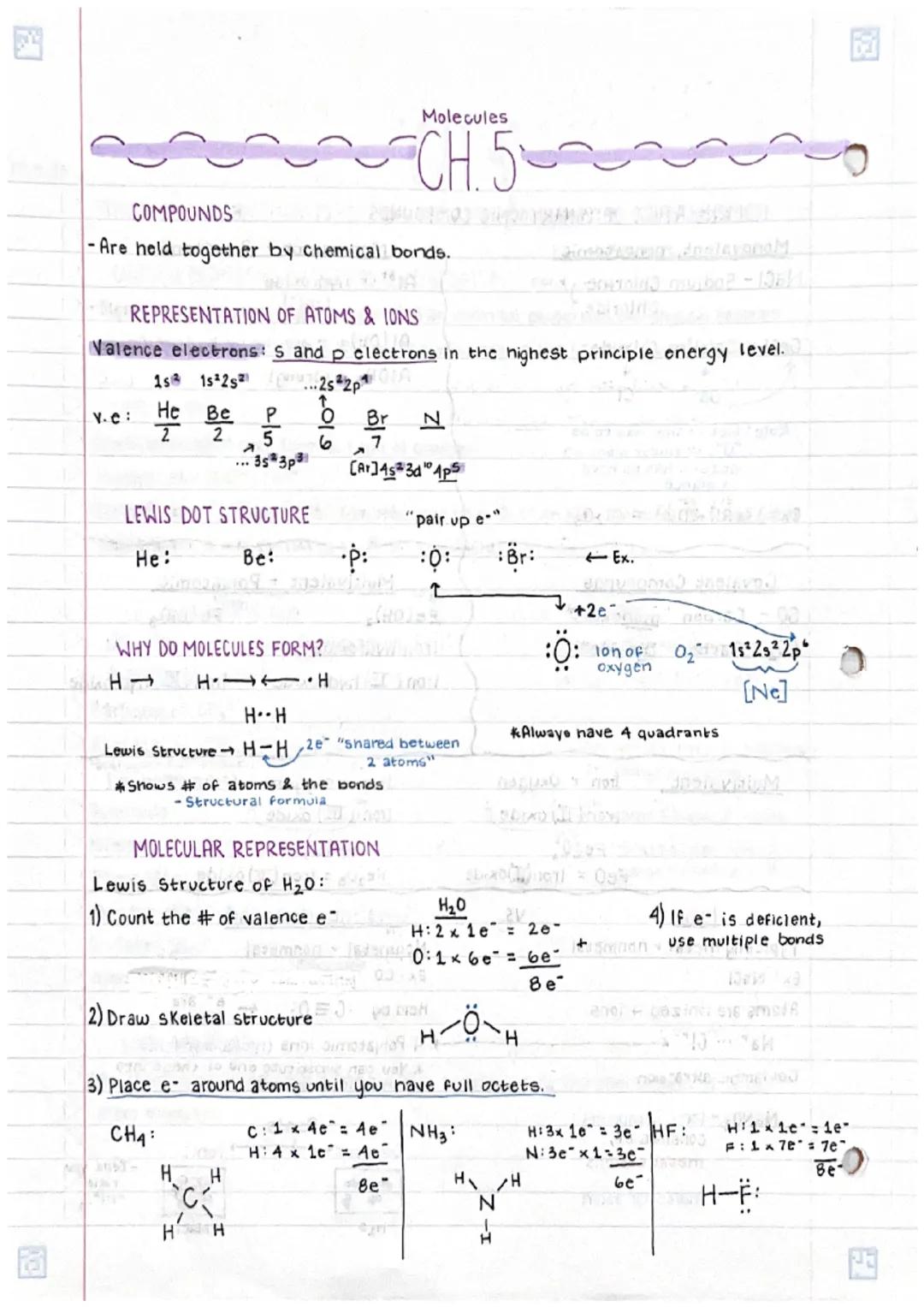
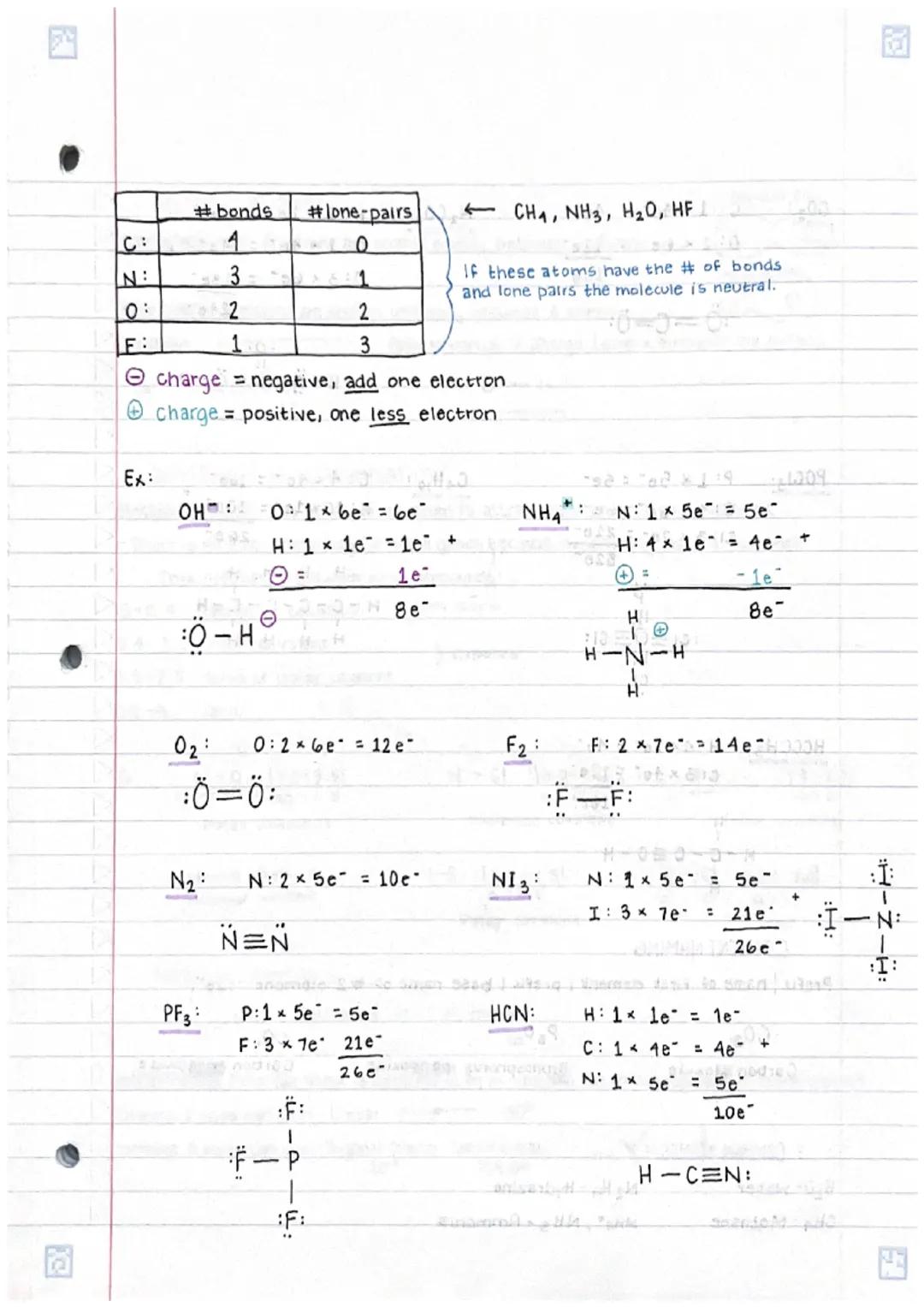
• Chemical compounds are held together through various types of bonds, with electrons playing a crucial role in bond formation
• Drawing Lewis dot structures for molecules involves systematic steps including counting valence electrons and arranging atoms to achieve stable octets
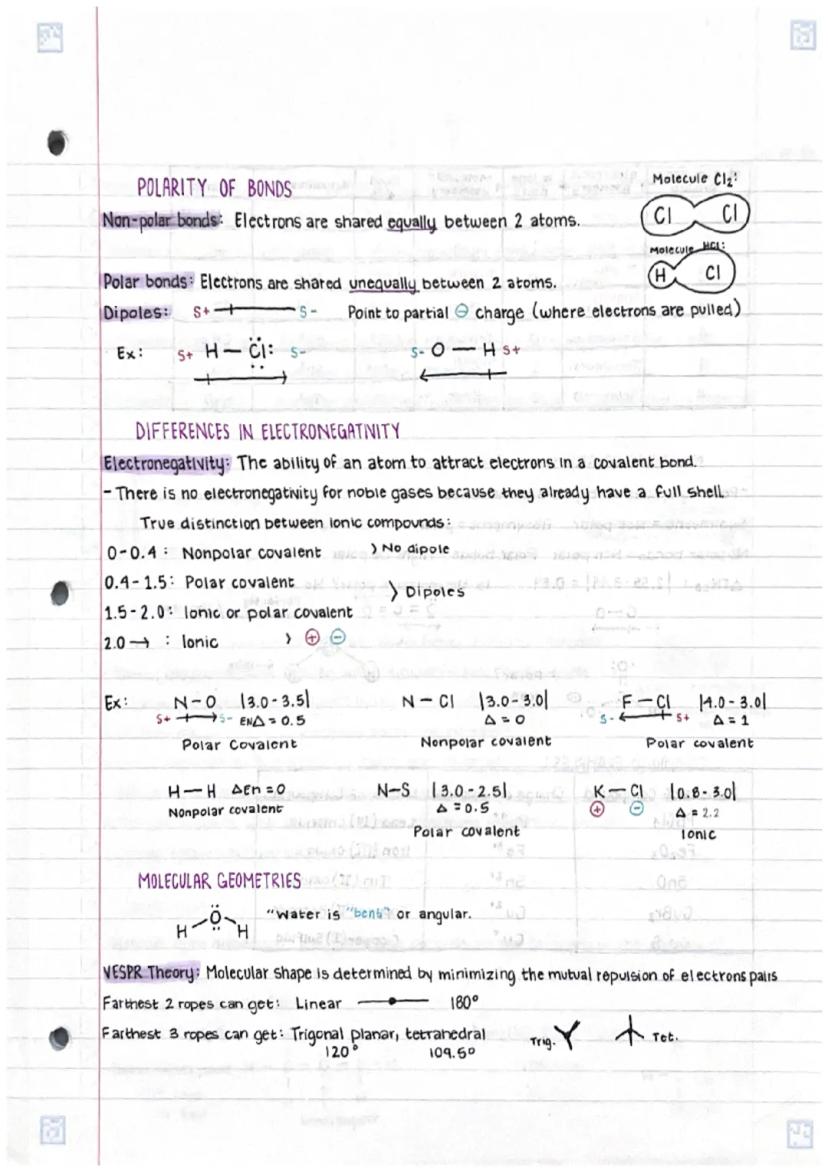
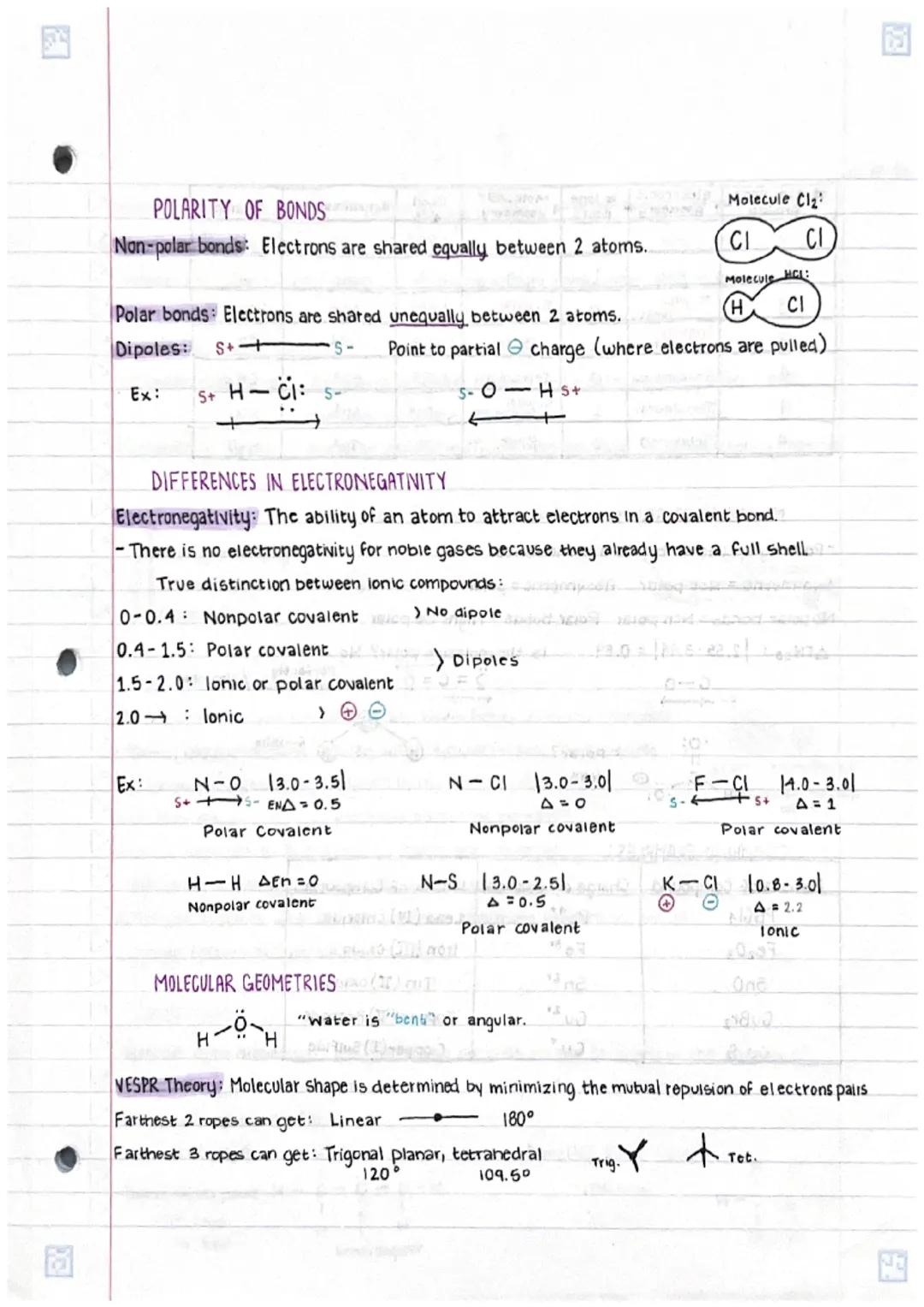
• Differences in polar and non-polar bonds arise from electronegativity variations between atoms, affecting molecular properties
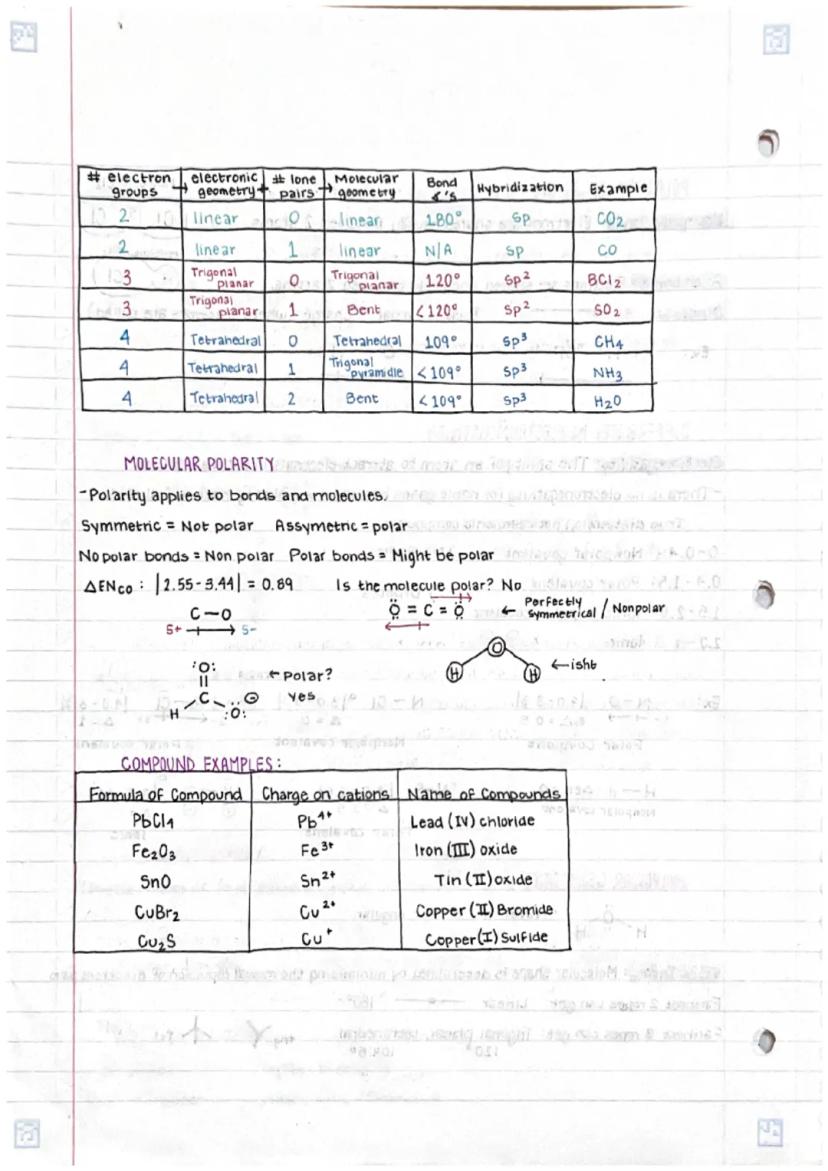
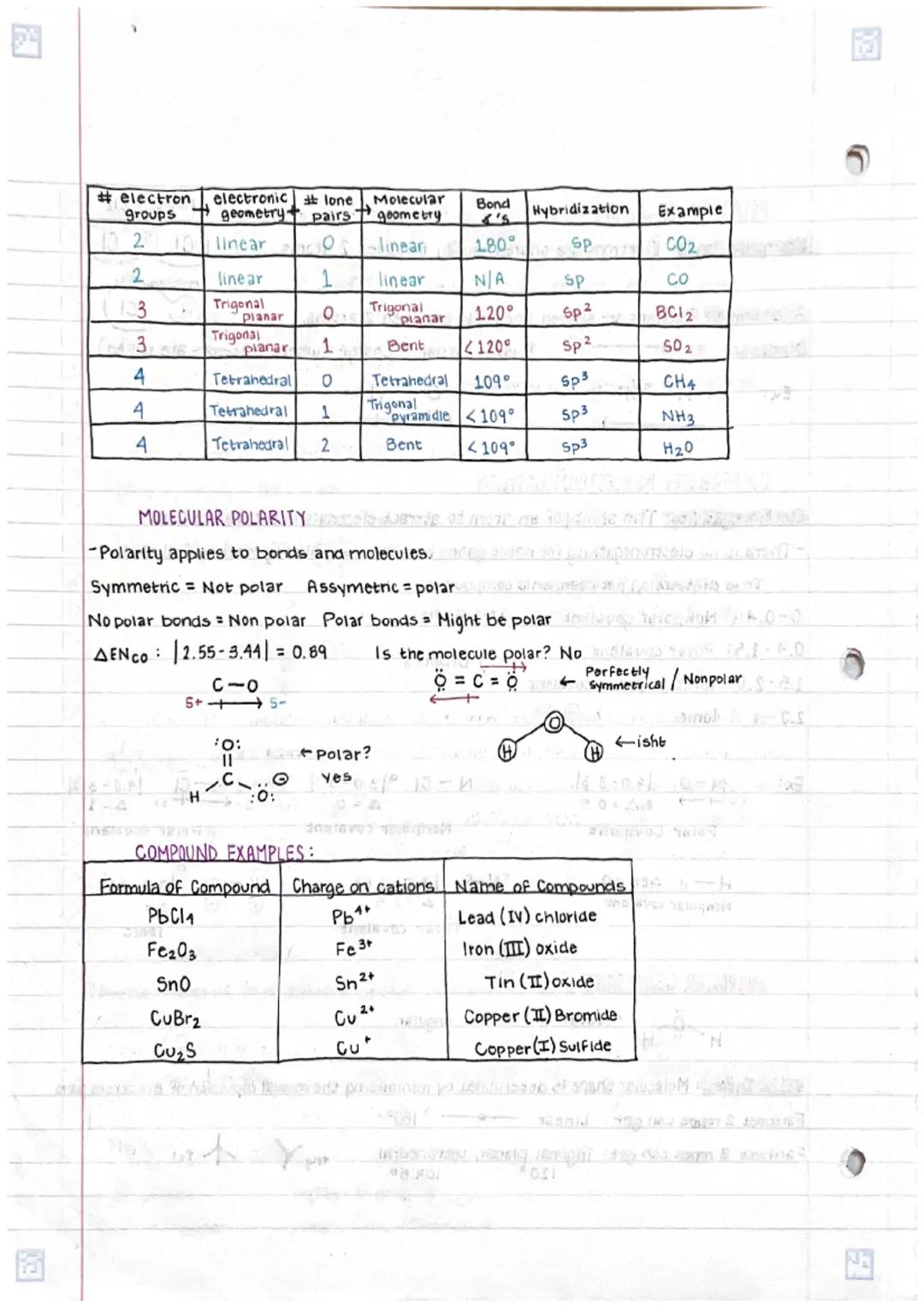
• Molecular geometry follows VSEPR theory principles, determining 3D shapes based on electron pair repulsions
• Bond polarity and molecular symmetry together determine overall molecular polarity