Dive into the fascinating world of biological molecules—the building blocks... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
105
•
Jan 31, 2026
•
Zuliana Loaiza
@ulianaoaiza_qxsb
Dive into the fascinating world of biological molecules—the building blocks... Show more






Ever wondered what you're actually made of at the molecular level? Your body is built from four types of biological macromolecules: carbohydrates, proteins, nucleic acids, and lipids. Most of these are polymers—long chains built from smaller repeating units called monomers.
The creation of these molecular chains happens through polymerization, where monomers are linked together through chemical reactions catalyzed by enzymes. Water plays a crucial role in this process: dehydration reactions (removing water) build polymers, while hydrolysis reactions (adding water) break them apart.
Carbohydrates start with simple sugars called monosaccharides. These include molecules with different numbers of carbon atoms: trioses (3 carbons), pentoses (5 carbons) like ribose, and hexoses (6 carbons) like glucose. Though often drawn in a linear form, these sugars actually exist as rings in your body.
Fun Fact: The slight differences in how hydroxyl groups are arranged in monosaccharides (alpha vs. beta forms) completely change how the resulting polymers behave in your body!
When two monosaccharides join together through a glycosidic bond, they form disaccharides like maltose , sucrose , and lactose . These are the sugars you encounter in everyday life!
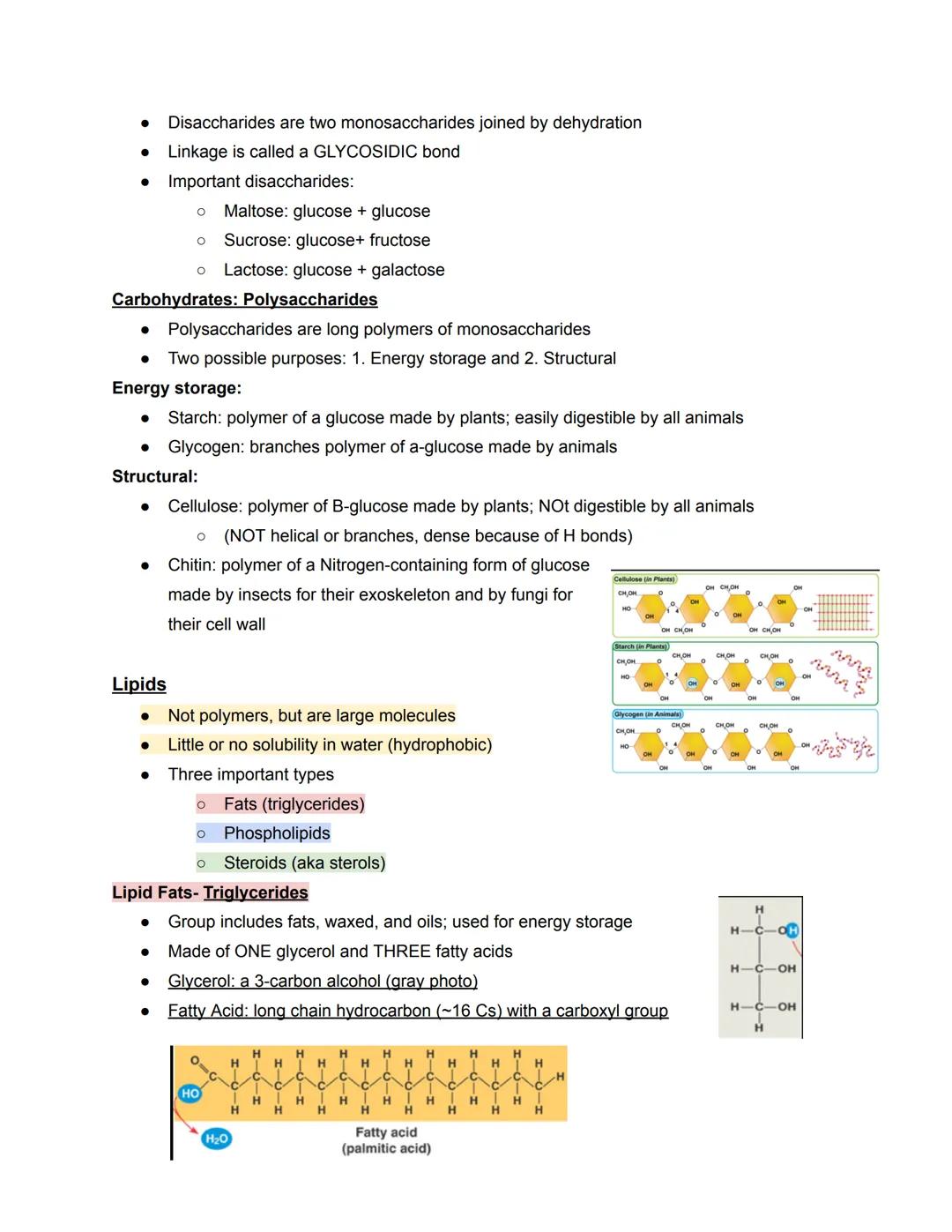
Longer chains form polysaccharides that serve two main functions: energy storage and structural support. Plants store energy as starch while animals use glycogen—both made of alpha-glucose. For structure, plants use cellulose , which gives them rigidity but can't be digested by most animals. Insects and fungi use chitin for their protective outer layers.
Unlike the other macromolecules, lipids aren't polymers but are still large, complex molecules. Their key characteristic is being hydrophobic—they don't mix with water. Fats (triglycerides) consist of one glycerol molecule attached to three fatty acids and function primarily for energy storage.
Remember This: The structural difference between saturated and unsaturated fats is crucial for your health! Unsaturated fats have double bonds that create "kinks" in their structure, making them liquid at room temperature and generally healthier for your body.
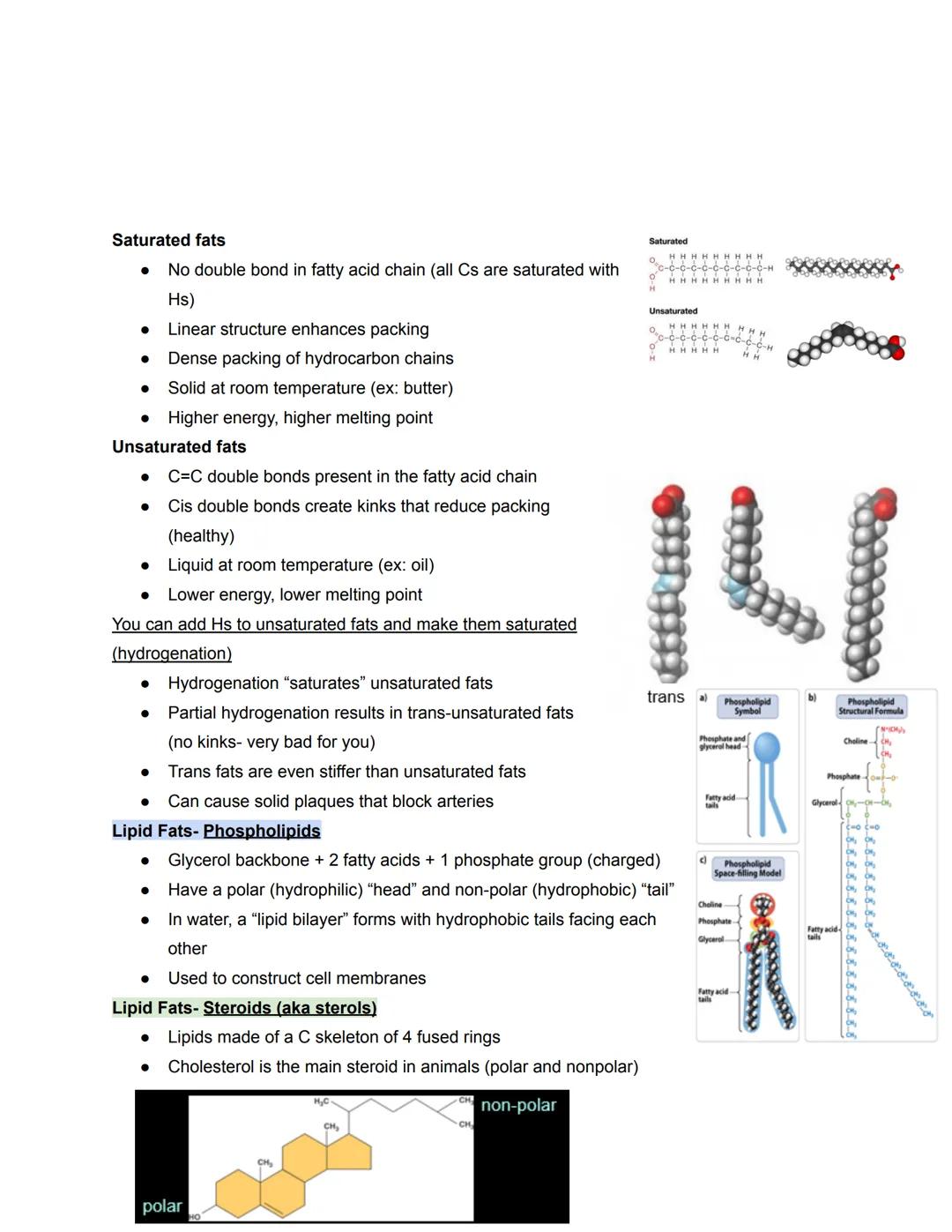
The structure of fatty acids dramatically affects their properties. Saturated fats have no double bonds, pack tightly together, and remain solid at room temperature (like butter). Their dense packing gives them higher energy content and higher melting points.
Unsaturated fats contain carbon-carbon double bonds that create kinks in their structure, preventing tight packing. This makes them liquid at room temperature (like oils) and generally healthier. Unfortunately, food manufacturers sometimes partially hydrogenate these fats, creating trans fats that lack the healthy kinks while maintaining stiffness—these can form dangerous arterial plaques.
Phospholipids represent a fascinating adaptation: they have a glycerol backbone with two fatty acids and one phosphate group. This gives them a dual nature—a water-loving (hydrophilic) head and water-avoiding (hydrophobic) tails. In water, they automatically arrange into lipid bilayers, the foundation of all cell membranes.
Steroids round out the lipid family with their distinctive structure of four fused carbon rings. Cholesterol is the primary steroid in animals and serves as a precursor to hormones like estrogen and testosterone while also being a crucial component of cell membranes.
Biology Insight: Your cell membranes are primarily phospholipid bilayers—this dual-nature molecule solved the fundamental challenge of creating a barrier that separates the watery environment inside cells from the watery environment outside!

Proteins are the workhorses of your cells, making up about 50% of their dry weight and performing an incredible diversity of functions. Each protein is a polypeptide—a polymer of amino acids folded into a specific functional shape. Remember: for proteins, shape equals function!
Your body uses 20 different amino acids to build its proteins. Each amino acid has the same basic structure: an amino group, a central carbon, a carboxyl group, and a unique side chain that gives it distinct chemical properties. These side chains can be non-polar (hydrophobic), polar (hydrophilic), or electrically charged (acidic or basic).
Amino acids link together through peptide bonds formed by dehydration reactions. New amino acids always add to the carboxyl end of the growing chain, creating a directional molecule with an amino group at one end and a carboxyl group at the other. The specific sequence of amino acids in a protein—determined by your genes—dictates how it will fold and function.
Test Tip: Understanding that a protein's function comes from its three-dimensional shape, which is determined by its amino acid sequence, is one of the most fundamental concepts in biology. This relationship between structure and function appears throughout biological systems!

Proteins perform an astonishing range of tasks in your body. Most function as enzymes that catalyze chemical reactions without being consumed themselves. Others provide structural support (like keratin in your hair), store amino acids, transport molecules across membranes, facilitate cell signaling (like insulin), enable muscle contraction, or provide immune defense.
Enzymes, the most abundant type of protein, work as biological catalysts by bringing reactants together in their active sites. Their names typically indicate their function—sucrase breaks down sucrose, DNAse breaks down DNA, and kinases add phosphate groups.
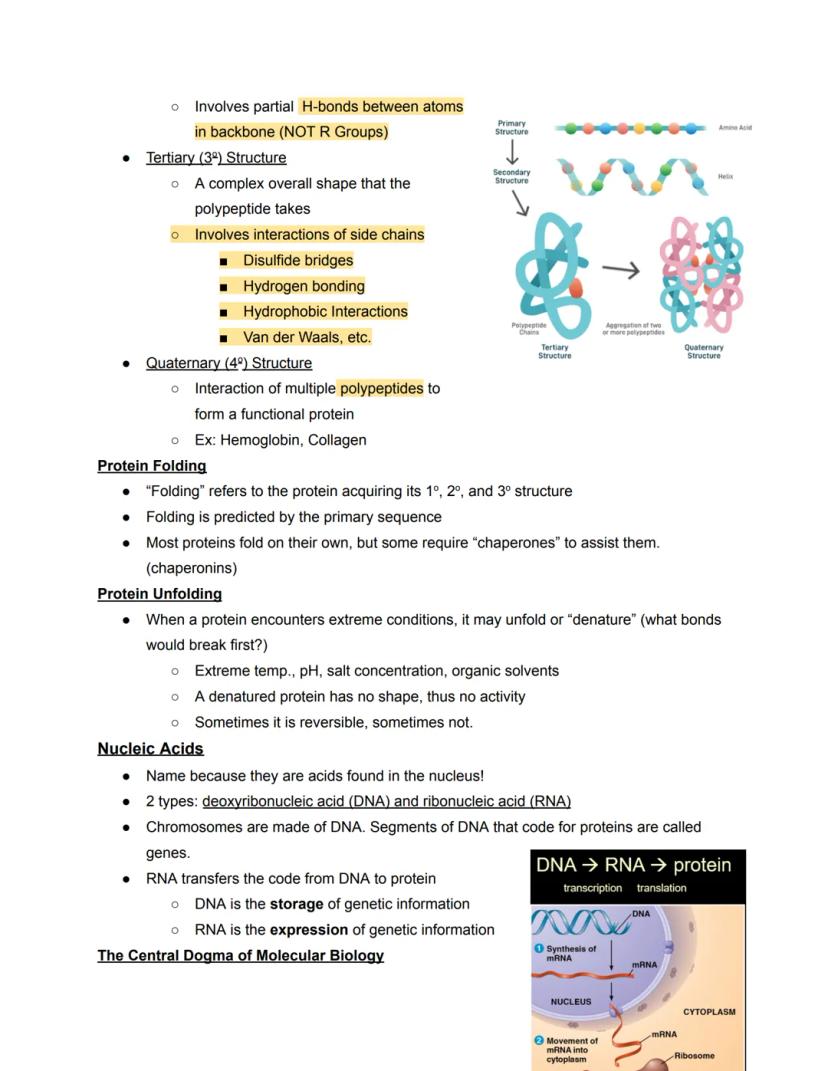
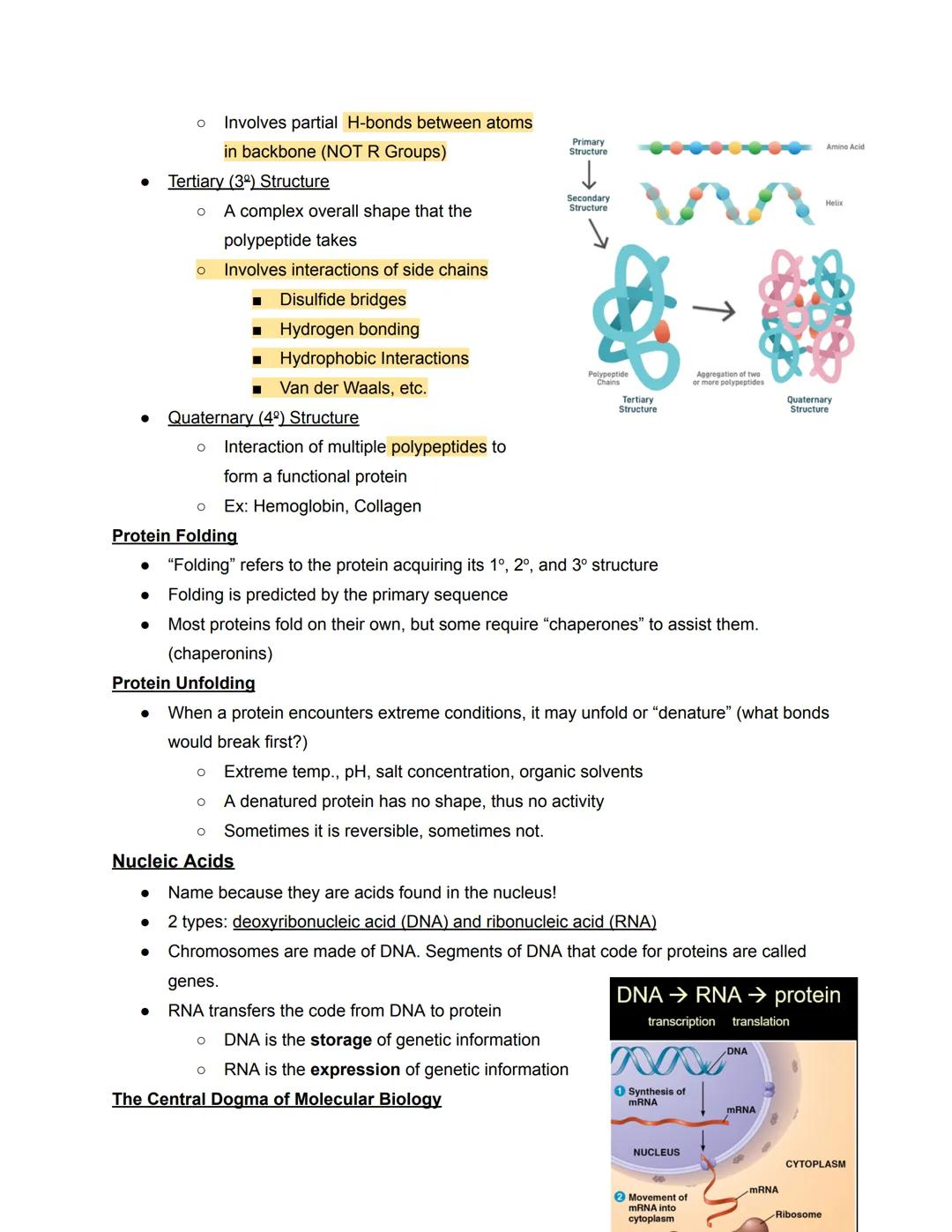
Protein structure is organized into four hierarchical levels. The primary structure is simply the sequence of amino acids, like a string of beads. The secondary structure involves local folding patterns called alpha-helices and beta-sheets, formed by hydrogen bonds between backbone atoms.
The tertiary structure represents the complete three-dimensional shape of a single polypeptide, held together by various interactions between side chains including disulfide bridges, hydrogen bonds, and hydrophobic interactions. When multiple polypeptides come together to form a functional protein, like hemoglobin, this creates the quaternary structure.
Critical Concept: A protein's primary structure (amino acid sequence) determines everything about how it will fold and function. If the sequence changes, even by a single amino acid, the protein's shape and function can be dramatically altered—this is how genetic mutations can cause diseases.
Protein folding is the process by which a linear chain of amino acids acquires its functional three-dimensional structure. Most proteins fold spontaneously based on their amino acid sequence, though some require helper proteins called chaperones to fold correctly.
When proteins encounter extreme conditions like high temperatures, abnormal pH, or certain chemicals, they can denature or unfold. Denaturation disrupts the protein's shape, destroying its function. Sometimes this process is reversible (like when egg whites cool after cooking), but often it's permanent.
Nucleic acids are the information-storing molecules of life. The two types—DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)—work together to transform genetic information into proteins. DNA, found primarily in the nucleus, stores your genetic information. RNA transfers this information from DNA to guide protein synthesis.
This information flow, called the Central Dogma of Molecular Biology, follows a specific path: DNA is transcribed into RNA, which is then translated into protein. The segments of DNA that code for specific proteins are called genes—these are your genetic instructions for building and maintaining your body.
Real-World Connection: When you cook an egg, the heat denatures the proteins, causing them to unfold and then re-form new bonds in a solid structure. This is why cooked eggs turn from clear to white and can't return to their original state!
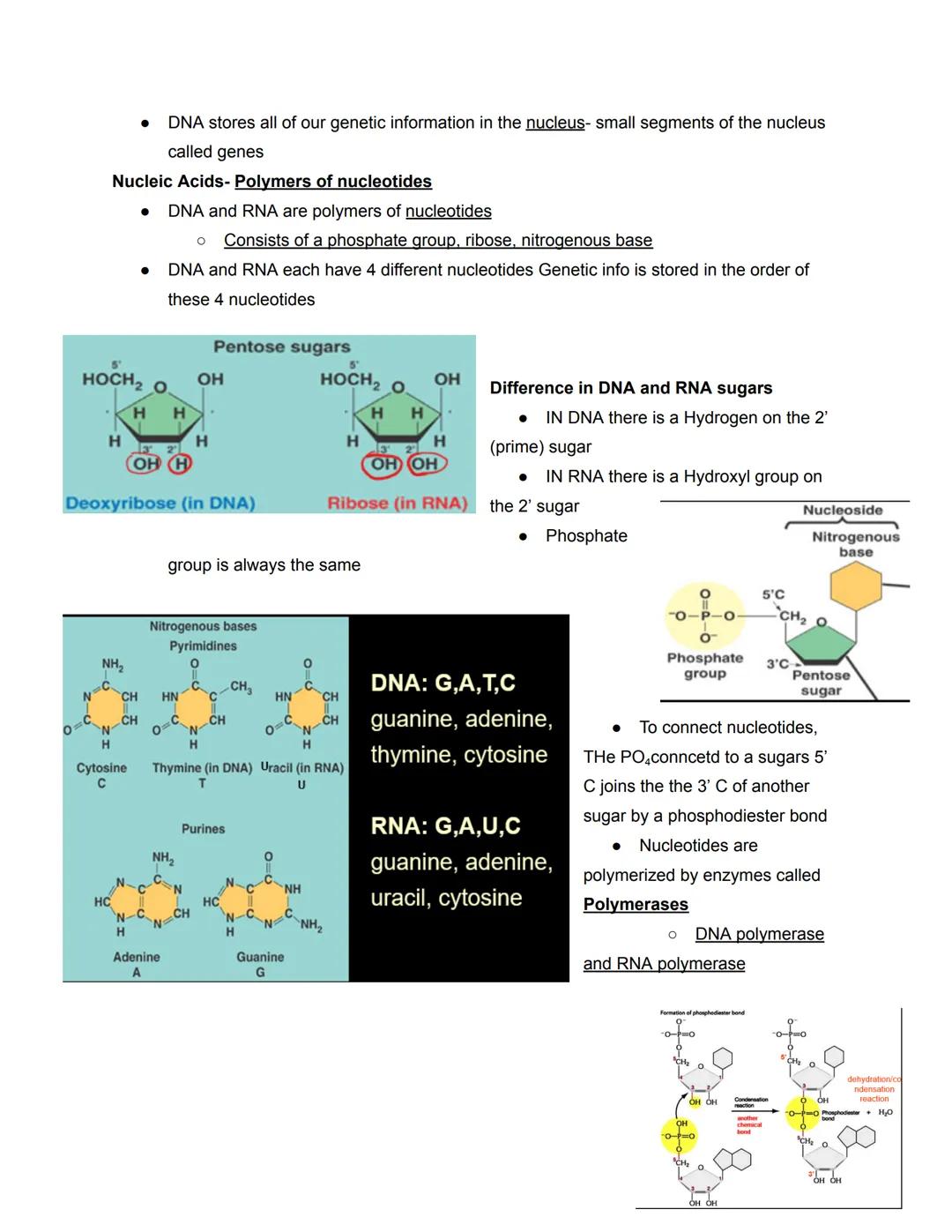
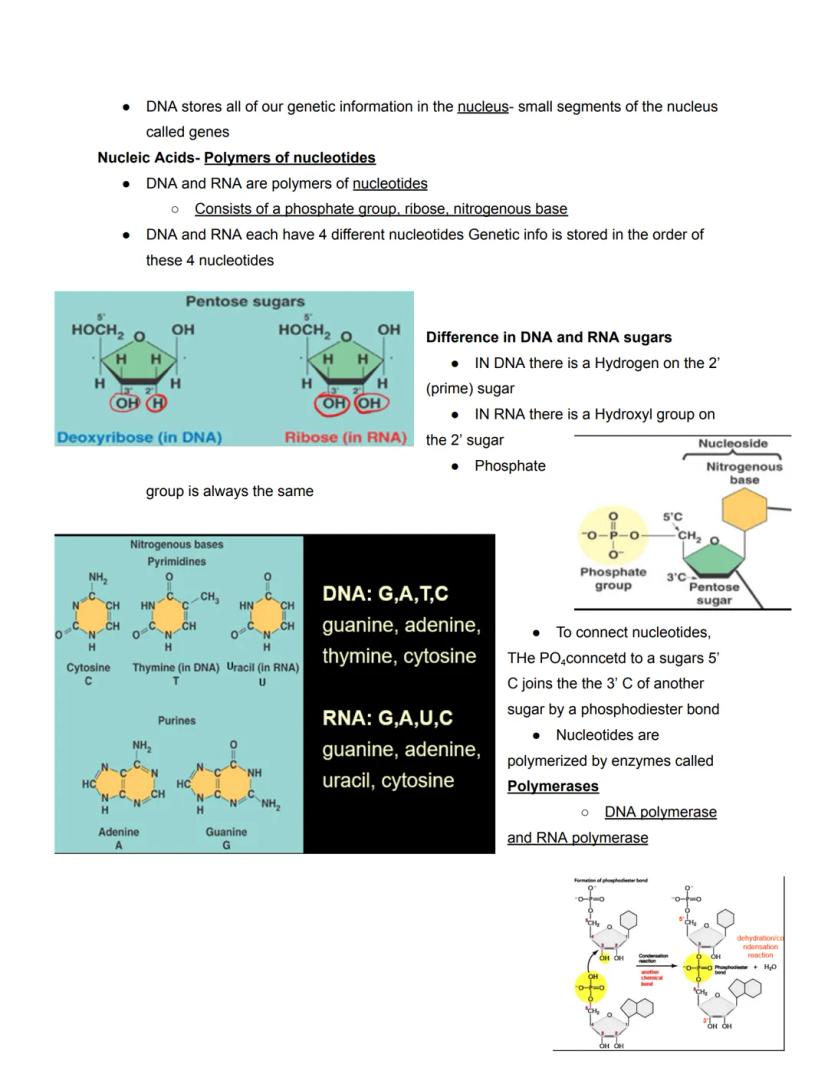
Both DNA and RNA are polymers made of subunits called nucleotides. Each nucleotide consists of three parts: a phosphate group, a five-carbon sugar (pentose), and a nitrogenous base. The genetic information is encoded in the sequence of these nucleotides.
DNA and RNA differ in several key ways. DNA contains the sugar deoxyribose (missing an oxygen at the 2' position), while RNA contains ribose. DNA uses the bases adenine (A), guanine (G), cytosine (C), and thymine (T), whereas RNA replaces thymine with uracil (U).
The nitrogenous bases come in two types: purines (A and G, with double rings) and pyrimidines (C, T, and U, with single rings). These bases are connected to the sugar, which is connected to the phosphate group. Nucleotides join together when the phosphate group attached to the 5' carbon of one sugar connects to the 3' carbon of another sugar, forming a phosphodiester bond.
Special enzymes called polymerases string nucleotides together to create nucleic acid polymers. DNA polymerase builds DNA strands, while RNA polymerase constructs RNA strands, both following the template of existing nucleic acids.
Visualization Tip: Think of nucleotides as building blocks with three parts: the phosphate group is the connector, the sugar forms the backbone, and the nitrogenous base is like a letter of a four-letter alphabet that spells out genetic instructions.
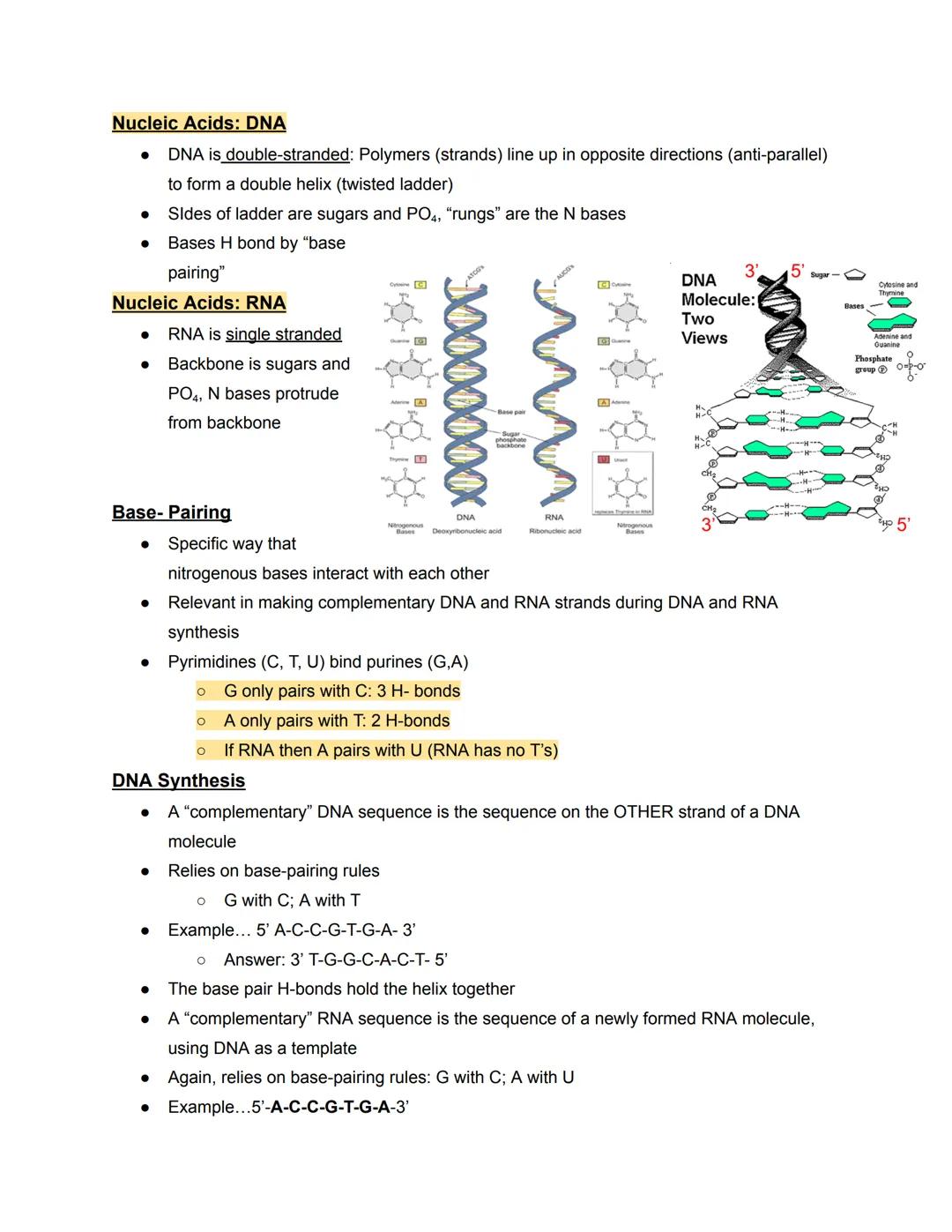
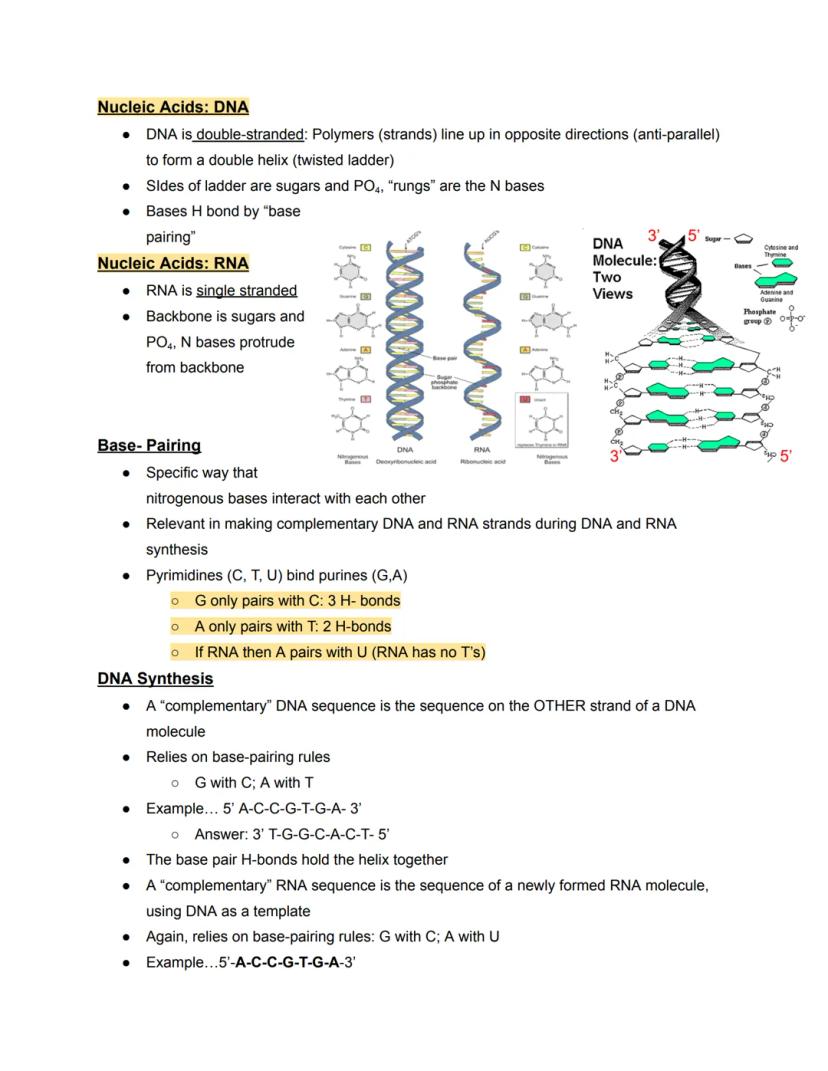
DNA's famous double helix structure resembles a twisted ladder. The sides of this ladder consist of alternating sugar and phosphate molecules, while the rungs are formed by paired nitrogenous bases held together by hydrogen bonds. The two strands run in opposite directions (antiparallel), with one strand running 5' to 3' and the other 3' to 5'.
Unlike DNA, RNA is typically single-stranded. It has a backbone of sugar and phosphate with nitrogenous bases extending from it, like a half-ladder. This structural difference allows RNA to fold into complex shapes that enable various functions.
Base-pairing follows specific rules essential for DNA replication and RNA synthesis. Purines always pair with pyrimidines: G bonds with C using three hydrogen bonds, while A bonds with T (in DNA) or U (in RNA) using two hydrogen bonds. This specific pairing ensures accurate copying of genetic information.
During DNA synthesis, the enzyme DNA polymerase uses an existing DNA strand as a template to create a complementary strand following these base-pairing rules. For example, if the template strand reads 5'-ACCGTGA-3', the new strand will be 3'-TGGCACT-5'. Similarly, during RNA synthesis (transcription), RNA polymerase creates an RNA strand complementary to a DNA template, substituting U for T.
Memory Aid: Remember base pairing with: "G hugs C tightly with three bonds, while A loosely embraces T (or U) with just two."
Let's pull everything together! The four major biological macromolecules can be summarized by their building blocks and how they connect:
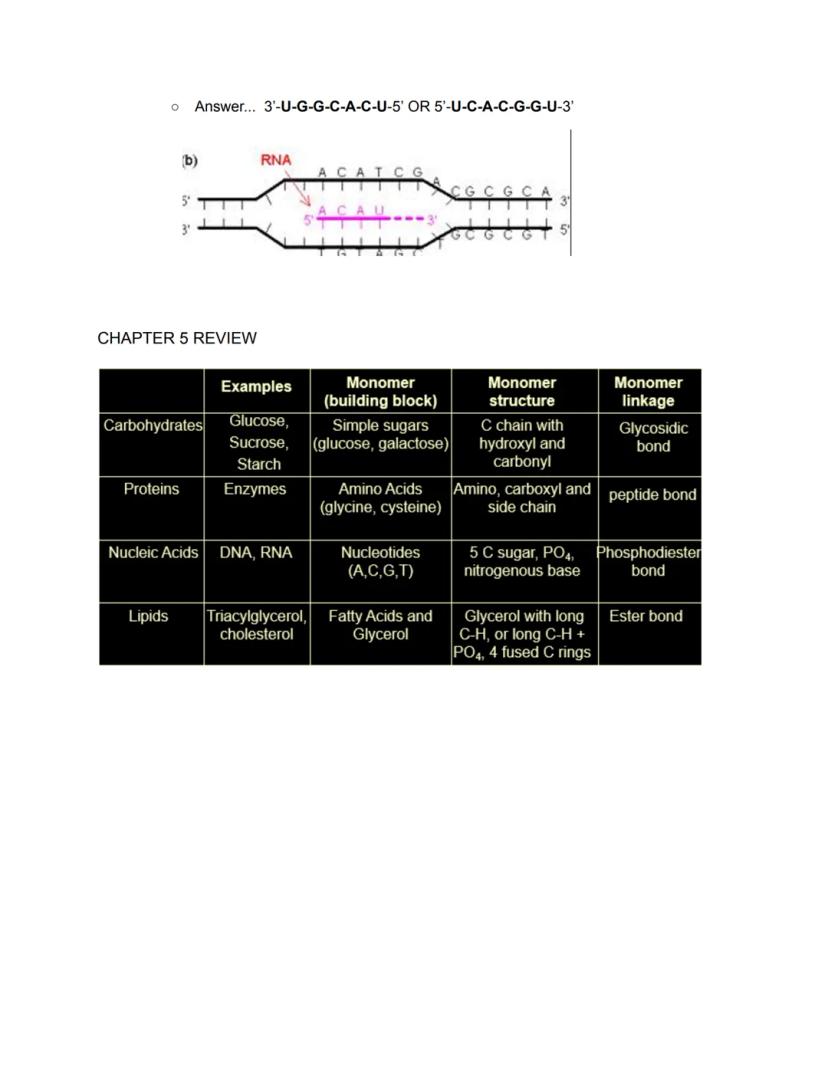
Carbohydrates like glucose, sucrose, and starch are built from simple sugar monomers (glucose, galactose) that have carbon chains with hydroxyl and carbonyl groups. These monomers connect through glycosidic bonds to form larger structures for energy storage and structural support.
Proteins, including all enzymes, are built from amino acid monomers (like glycine and cysteine), each with amino, carboxyl, and unique side chain groups. These connect via peptide bonds to form chains that fold into functional shapes that perform virtually all cellular work.
Nucleic acids (DNA and RNA) are formed from nucleotide monomers that contain a 5-carbon sugar, phosphate group, and nitrogenous base (A, C, G, T or U). These join through phosphodiester bonds to create the molecules that store and transmit genetic information.
Lipids like triglycerides and cholesterol aren't true polymers but include fatty acids (long hydrocarbon chains) often connected to glycerol by ester bonds. These form diverse structures that store energy, build membranes, and function as hormones.
Big Picture: Every biological function in your body—from thinking to growing to fighting disease—depends on the precise structure and interaction of these four types of macromolecules. Their diversity explains how the same basic elements (C, H, O, N) can create the incredible complexity of life!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Zuliana Loaiza
@ulianaoaiza_qxsb
Dive into the fascinating world of biological molecules—the building blocks of all living things. Chapter 5 explores four key macromolecules (carbohydrates, proteins, nucleic acids, and lipids) that make life possible, showing how their structure determines their function in our bodies.

Access to all documents
Improve your grades
Join milions of students
Ever wondered what you're actually made of at the molecular level? Your body is built from four types of biological macromolecules: carbohydrates, proteins, nucleic acids, and lipids. Most of these are polymers—long chains built from smaller repeating units called monomers.
The creation of these molecular chains happens through polymerization, where monomers are linked together through chemical reactions catalyzed by enzymes. Water plays a crucial role in this process: dehydration reactions (removing water) build polymers, while hydrolysis reactions (adding water) break them apart.
Carbohydrates start with simple sugars called monosaccharides. These include molecules with different numbers of carbon atoms: trioses (3 carbons), pentoses (5 carbons) like ribose, and hexoses (6 carbons) like glucose. Though often drawn in a linear form, these sugars actually exist as rings in your body.
Fun Fact: The slight differences in how hydroxyl groups are arranged in monosaccharides (alpha vs. beta forms) completely change how the resulting polymers behave in your body!

Access to all documents
Improve your grades
Join milions of students
When two monosaccharides join together through a glycosidic bond, they form disaccharides like maltose , sucrose , and lactose . These are the sugars you encounter in everyday life!
Longer chains form polysaccharides that serve two main functions: energy storage and structural support. Plants store energy as starch while animals use glycogen—both made of alpha-glucose. For structure, plants use cellulose , which gives them rigidity but can't be digested by most animals. Insects and fungi use chitin for their protective outer layers.
Unlike the other macromolecules, lipids aren't polymers but are still large, complex molecules. Their key characteristic is being hydrophobic—they don't mix with water. Fats (triglycerides) consist of one glycerol molecule attached to three fatty acids and function primarily for energy storage.
Remember This: The structural difference between saturated and unsaturated fats is crucial for your health! Unsaturated fats have double bonds that create "kinks" in their structure, making them liquid at room temperature and generally healthier for your body.

Access to all documents
Improve your grades
Join milions of students
The structure of fatty acids dramatically affects their properties. Saturated fats have no double bonds, pack tightly together, and remain solid at room temperature (like butter). Their dense packing gives them higher energy content and higher melting points.
Unsaturated fats contain carbon-carbon double bonds that create kinks in their structure, preventing tight packing. This makes them liquid at room temperature (like oils) and generally healthier. Unfortunately, food manufacturers sometimes partially hydrogenate these fats, creating trans fats that lack the healthy kinks while maintaining stiffness—these can form dangerous arterial plaques.
Phospholipids represent a fascinating adaptation: they have a glycerol backbone with two fatty acids and one phosphate group. This gives them a dual nature—a water-loving (hydrophilic) head and water-avoiding (hydrophobic) tails. In water, they automatically arrange into lipid bilayers, the foundation of all cell membranes.
Steroids round out the lipid family with their distinctive structure of four fused carbon rings. Cholesterol is the primary steroid in animals and serves as a precursor to hormones like estrogen and testosterone while also being a crucial component of cell membranes.
Biology Insight: Your cell membranes are primarily phospholipid bilayers—this dual-nature molecule solved the fundamental challenge of creating a barrier that separates the watery environment inside cells from the watery environment outside!

Access to all documents
Improve your grades
Join milions of students
Proteins are the workhorses of your cells, making up about 50% of their dry weight and performing an incredible diversity of functions. Each protein is a polypeptide—a polymer of amino acids folded into a specific functional shape. Remember: for proteins, shape equals function!
Your body uses 20 different amino acids to build its proteins. Each amino acid has the same basic structure: an amino group, a central carbon, a carboxyl group, and a unique side chain that gives it distinct chemical properties. These side chains can be non-polar (hydrophobic), polar (hydrophilic), or electrically charged (acidic or basic).
Amino acids link together through peptide bonds formed by dehydration reactions. New amino acids always add to the carboxyl end of the growing chain, creating a directional molecule with an amino group at one end and a carboxyl group at the other. The specific sequence of amino acids in a protein—determined by your genes—dictates how it will fold and function.
Test Tip: Understanding that a protein's function comes from its three-dimensional shape, which is determined by its amino acid sequence, is one of the most fundamental concepts in biology. This relationship between structure and function appears throughout biological systems!

Access to all documents
Improve your grades
Join milions of students
Proteins perform an astonishing range of tasks in your body. Most function as enzymes that catalyze chemical reactions without being consumed themselves. Others provide structural support (like keratin in your hair), store amino acids, transport molecules across membranes, facilitate cell signaling (like insulin), enable muscle contraction, or provide immune defense.
Enzymes, the most abundant type of protein, work as biological catalysts by bringing reactants together in their active sites. Their names typically indicate their function—sucrase breaks down sucrose, DNAse breaks down DNA, and kinases add phosphate groups.
Protein structure is organized into four hierarchical levels. The primary structure is simply the sequence of amino acids, like a string of beads. The secondary structure involves local folding patterns called alpha-helices and beta-sheets, formed by hydrogen bonds between backbone atoms.
The tertiary structure represents the complete three-dimensional shape of a single polypeptide, held together by various interactions between side chains including disulfide bridges, hydrogen bonds, and hydrophobic interactions. When multiple polypeptides come together to form a functional protein, like hemoglobin, this creates the quaternary structure.
Critical Concept: A protein's primary structure (amino acid sequence) determines everything about how it will fold and function. If the sequence changes, even by a single amino acid, the protein's shape and function can be dramatically altered—this is how genetic mutations can cause diseases.

Access to all documents
Improve your grades
Join milions of students
Protein folding is the process by which a linear chain of amino acids acquires its functional three-dimensional structure. Most proteins fold spontaneously based on their amino acid sequence, though some require helper proteins called chaperones to fold correctly.
When proteins encounter extreme conditions like high temperatures, abnormal pH, or certain chemicals, they can denature or unfold. Denaturation disrupts the protein's shape, destroying its function. Sometimes this process is reversible (like when egg whites cool after cooking), but often it's permanent.
Nucleic acids are the information-storing molecules of life. The two types—DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)—work together to transform genetic information into proteins. DNA, found primarily in the nucleus, stores your genetic information. RNA transfers this information from DNA to guide protein synthesis.
This information flow, called the Central Dogma of Molecular Biology, follows a specific path: DNA is transcribed into RNA, which is then translated into protein. The segments of DNA that code for specific proteins are called genes—these are your genetic instructions for building and maintaining your body.
Real-World Connection: When you cook an egg, the heat denatures the proteins, causing them to unfold and then re-form new bonds in a solid structure. This is why cooked eggs turn from clear to white and can't return to their original state!

Access to all documents
Improve your grades
Join milions of students
Both DNA and RNA are polymers made of subunits called nucleotides. Each nucleotide consists of three parts: a phosphate group, a five-carbon sugar (pentose), and a nitrogenous base. The genetic information is encoded in the sequence of these nucleotides.
DNA and RNA differ in several key ways. DNA contains the sugar deoxyribose (missing an oxygen at the 2' position), while RNA contains ribose. DNA uses the bases adenine (A), guanine (G), cytosine (C), and thymine (T), whereas RNA replaces thymine with uracil (U).
The nitrogenous bases come in two types: purines (A and G, with double rings) and pyrimidines (C, T, and U, with single rings). These bases are connected to the sugar, which is connected to the phosphate group. Nucleotides join together when the phosphate group attached to the 5' carbon of one sugar connects to the 3' carbon of another sugar, forming a phosphodiester bond.
Special enzymes called polymerases string nucleotides together to create nucleic acid polymers. DNA polymerase builds DNA strands, while RNA polymerase constructs RNA strands, both following the template of existing nucleic acids.
Visualization Tip: Think of nucleotides as building blocks with three parts: the phosphate group is the connector, the sugar forms the backbone, and the nitrogenous base is like a letter of a four-letter alphabet that spells out genetic instructions.

Access to all documents
Improve your grades
Join milions of students
DNA's famous double helix structure resembles a twisted ladder. The sides of this ladder consist of alternating sugar and phosphate molecules, while the rungs are formed by paired nitrogenous bases held together by hydrogen bonds. The two strands run in opposite directions (antiparallel), with one strand running 5' to 3' and the other 3' to 5'.
Unlike DNA, RNA is typically single-stranded. It has a backbone of sugar and phosphate with nitrogenous bases extending from it, like a half-ladder. This structural difference allows RNA to fold into complex shapes that enable various functions.
Base-pairing follows specific rules essential for DNA replication and RNA synthesis. Purines always pair with pyrimidines: G bonds with C using three hydrogen bonds, while A bonds with T (in DNA) or U (in RNA) using two hydrogen bonds. This specific pairing ensures accurate copying of genetic information.
During DNA synthesis, the enzyme DNA polymerase uses an existing DNA strand as a template to create a complementary strand following these base-pairing rules. For example, if the template strand reads 5'-ACCGTGA-3', the new strand will be 3'-TGGCACT-5'. Similarly, during RNA synthesis (transcription), RNA polymerase creates an RNA strand complementary to a DNA template, substituting U for T.
Memory Aid: Remember base pairing with: "G hugs C tightly with three bonds, while A loosely embraces T (or U) with just two."

Access to all documents
Improve your grades
Join milions of students
Let's pull everything together! The four major biological macromolecules can be summarized by their building blocks and how they connect:
Carbohydrates like glucose, sucrose, and starch are built from simple sugar monomers (glucose, galactose) that have carbon chains with hydroxyl and carbonyl groups. These monomers connect through glycosidic bonds to form larger structures for energy storage and structural support.
Proteins, including all enzymes, are built from amino acid monomers (like glycine and cysteine), each with amino, carboxyl, and unique side chain groups. These connect via peptide bonds to form chains that fold into functional shapes that perform virtually all cellular work.
Nucleic acids (DNA and RNA) are formed from nucleotide monomers that contain a 5-carbon sugar, phosphate group, and nitrogenous base (A, C, G, T or U). These join through phosphodiester bonds to create the molecules that store and transmit genetic information.
Lipids like triglycerides and cholesterol aren't true polymers but include fatty acids (long hydrocarbon chains) often connected to glycerol by ester bonds. These form diverse structures that store energy, build membranes, and function as hormones.
Big Picture: Every biological function in your body—from thinking to growing to fighting disease—depends on the precise structure and interaction of these four types of macromolecules. Their diversity explains how the same basic elements (C, H, O, N) can create the incredible complexity of life!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
11
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user