Carbon is the backbone of all life on Earth. Its... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Knowunity AI
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
53
•
Feb 7, 2026
•
Jasy Rodriguez
@jasyrodriguez_fpij
Carbon is the backbone of all life on Earth. Its... Show more



Carbon is truly remarkable - it forms the foundation for all biological molecules in every living thing on our planet. This versatile element creates an astonishing range of compounds that give life its incredible diversity.
Think of carbon as the alphabet of life - just as 26 letters can form countless words, carbon atoms can arrange themselves into millions of different molecules with unique functions.
Fun Fact: The study of carbon compounds is called organic chemistry because these molecules were once thought to exist only within living organisms!

Ever wonder what you're actually made of? At the molecular level, you're primarily carbon-based! All living organisms are built from chemical compounds that center around carbon atoms.
Organic chemistry specifically studies carbon compounds. This field explores how carbon's unique properties allow it to create the building blocks of life - from simple sugars to complex proteins that make you who you are.
When scientists analyze living tissues, they find carbon everywhere. It's in your DNA, proteins, fats, and even the molecules that provide energy to your cells.
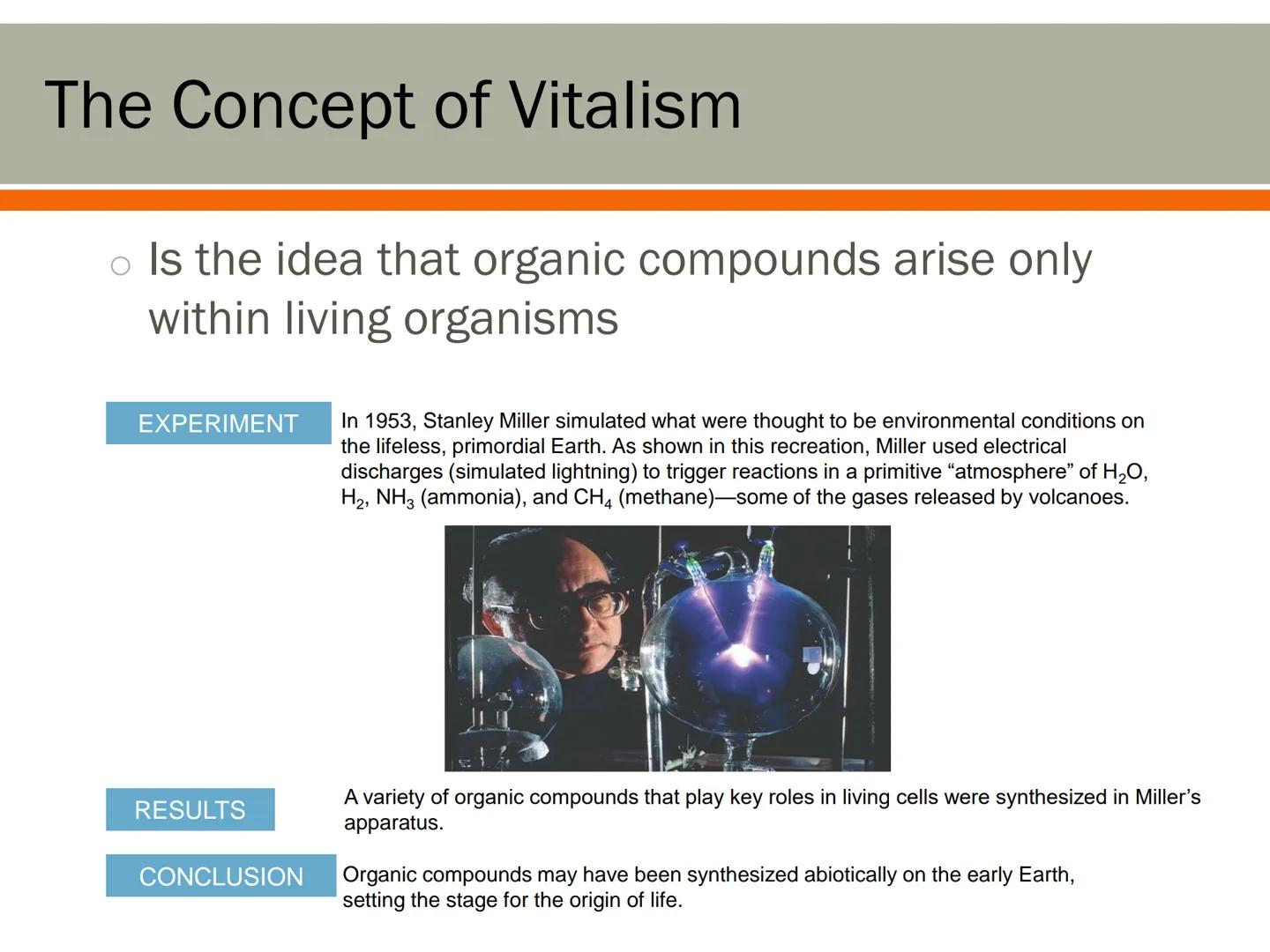
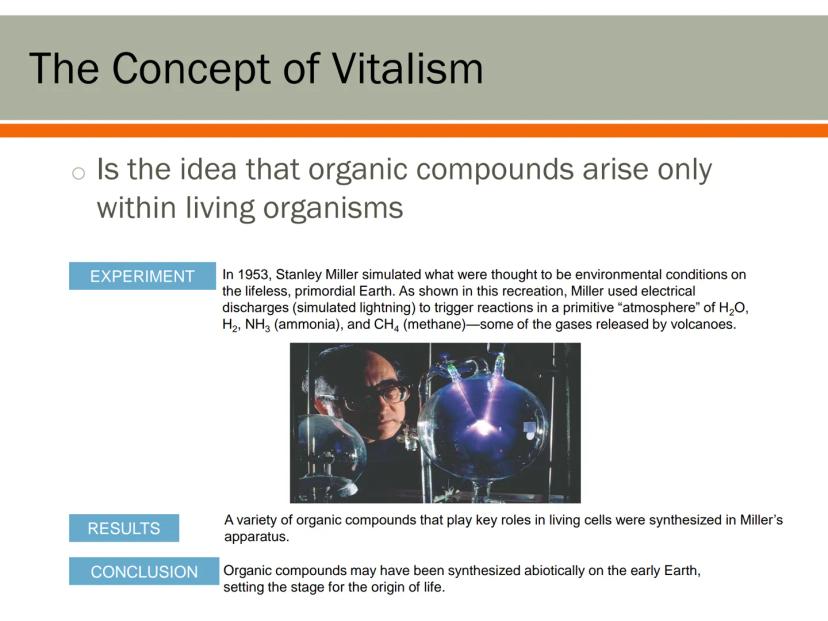
People once believed that organic compounds could only be created inside living things - an idea called vitalism. They thought living organisms had a special "vital force" that non-living things lacked.
This idea was challenged in a groundbreaking 1953 experiment by Stanley Miller. He recreated what scientists believed were Earth's early conditions - water vapor, hydrogen, ammonia, and methane - and added electrical sparks to simulate lightning.
The results were amazing! Miller's experiment produced several organic compounds essential for life, including amino acids (the building blocks of proteins). This suggested that the molecules needed for life could form naturally without any "vital force."
Mind-Blowing: Miller's experiment showed that the basic ingredients for life could have formed on early Earth through natural chemical reactions, possibly setting the stage for life's beginnings!
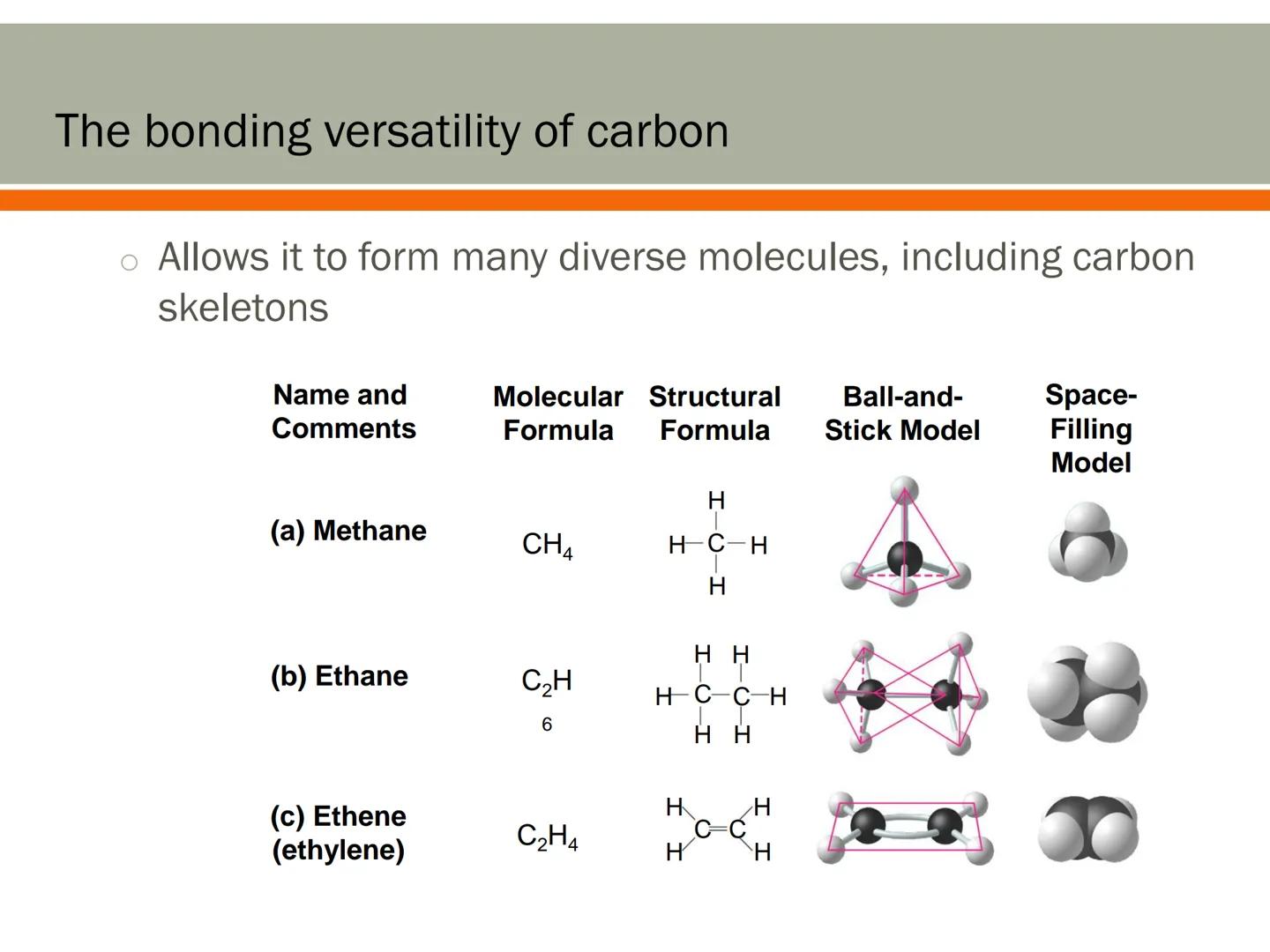
Carbon's superpower is its ability to form bonds with up to four other atoms simultaneously. This makes it incredibly versatile compared to most other elements.
Why can carbon form four bonds? It has four valence electrons (electrons in its outer shell) that can be shared with other atoms to form stable covalent bonds.
This four-bond capability is like having four arms to connect with different partners. Carbon can bond with various elements including hydrogen, oxygen, nitrogen, and even other carbon atoms. This flexibility allows carbon to create chains, rings, and branches - the essential frameworks for biological molecules.
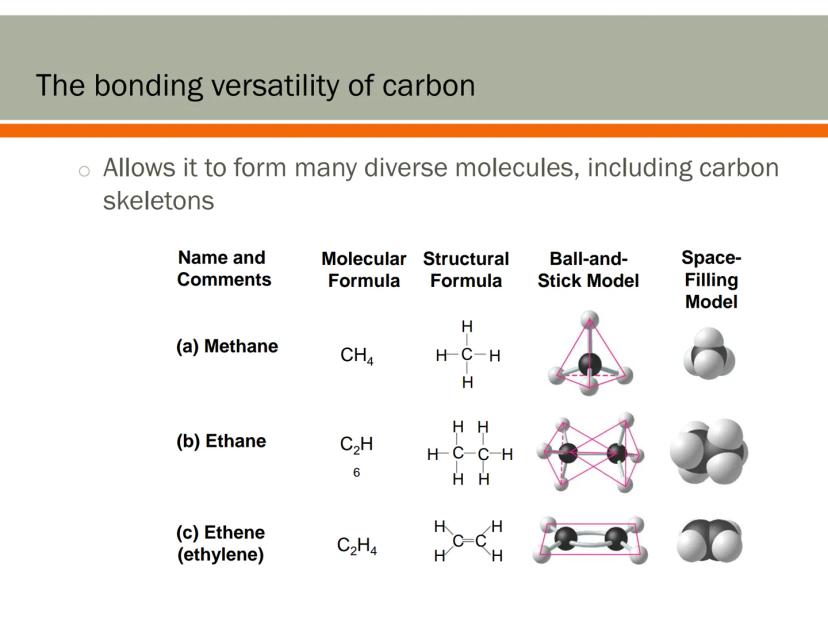
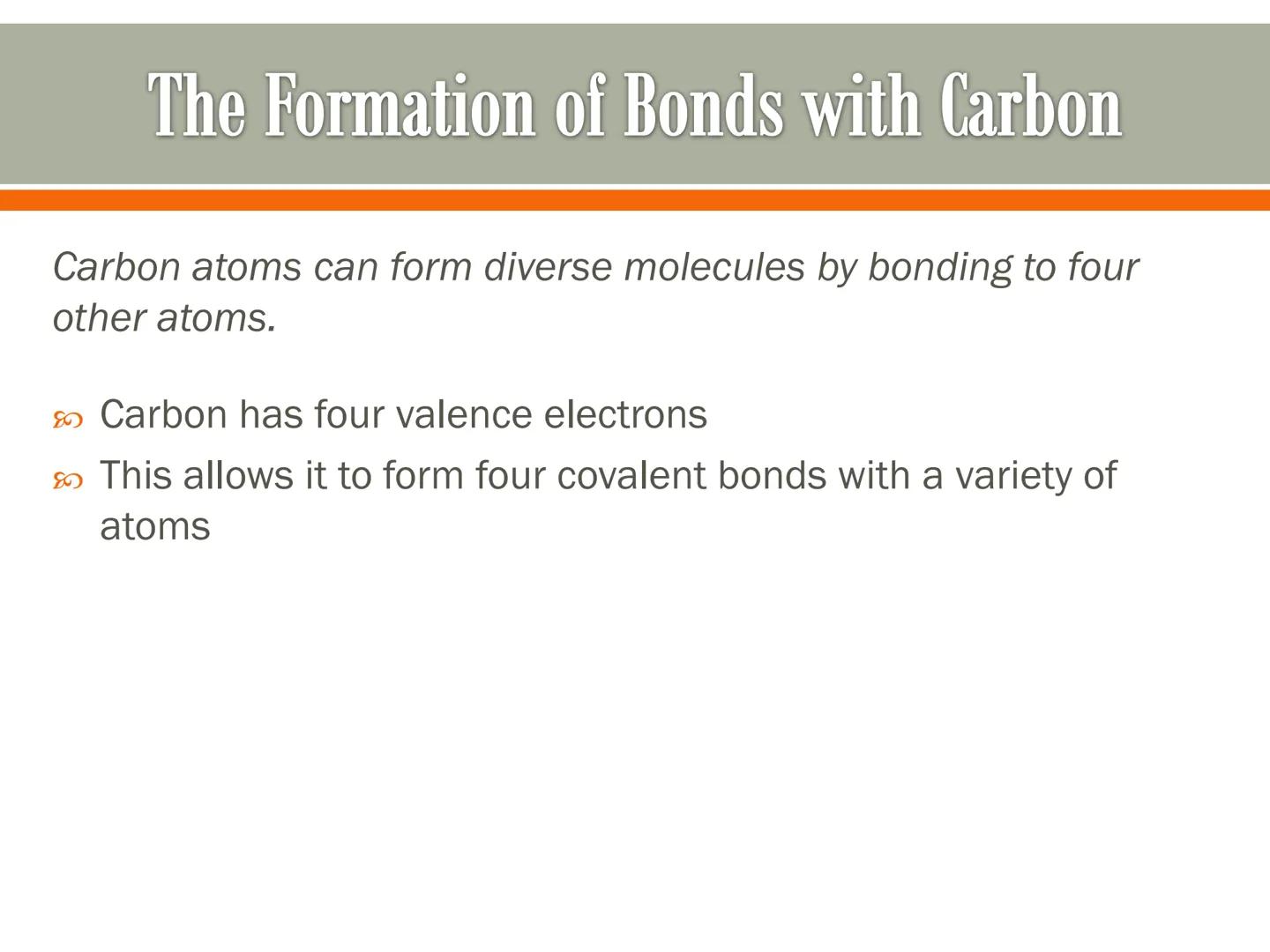
Carbon's bonding abilities allow it to form incredibly diverse molecular structures. These carbon skeletons serve as frameworks for the molecules that make up your body.
Simple carbon compounds like methane (CH₄) have just one carbon atom bonded to four hydrogen atoms. More complex molecules like ethane (C₂H₆) feature carbon atoms bonded to each other, creating chains.
Carbon can also form double bonds, as seen in ethene (C₂H₄). These double bonds create different shapes and properties in molecules, adding to the diversity of possible structures.
Chemistry Hack: When visualizing molecules, different models help show different aspects - structural formulas show the bonds, while space-filling models show the actual shape and size!
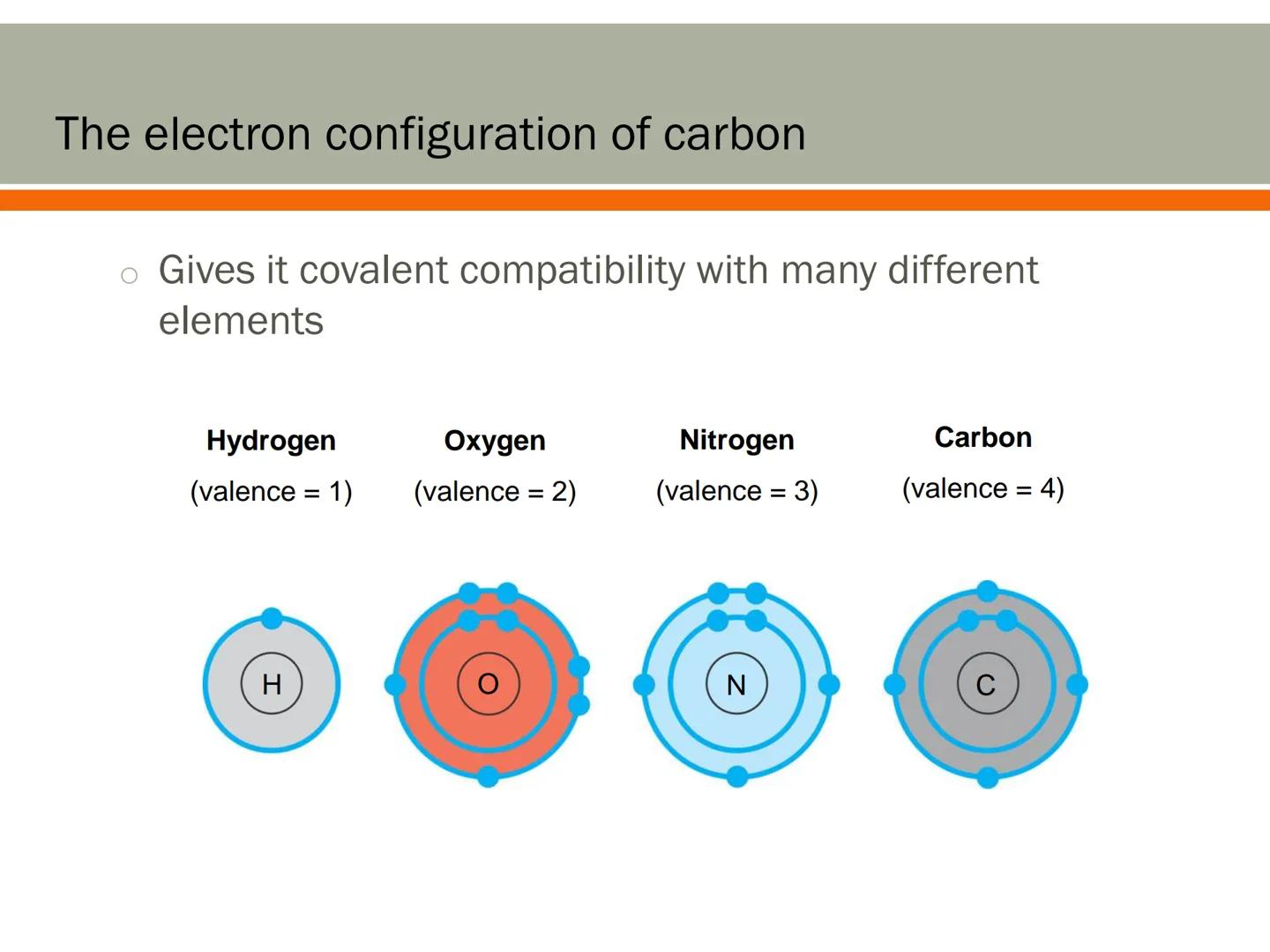
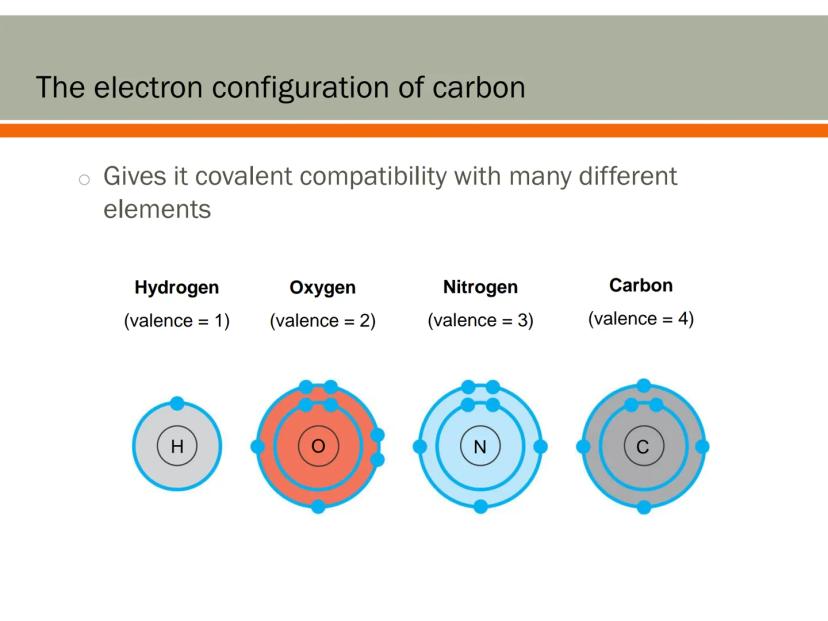
Carbon's electron configuration makes it extremely compatible with different elements. This compatibility is key to creating the diverse molecules found in living organisms.
Carbon readily forms bonds with hydrogen (which forms 1 bond), oxygen (which forms 2 bonds), and nitrogen (which forms 3 bonds). This allows carbon to be the central connecting element in most biological molecules.
Think of carbon as the ultimate team player in the molecular world. It can connect with almost any other element, creating countless combinations that serve different functions in living systems.
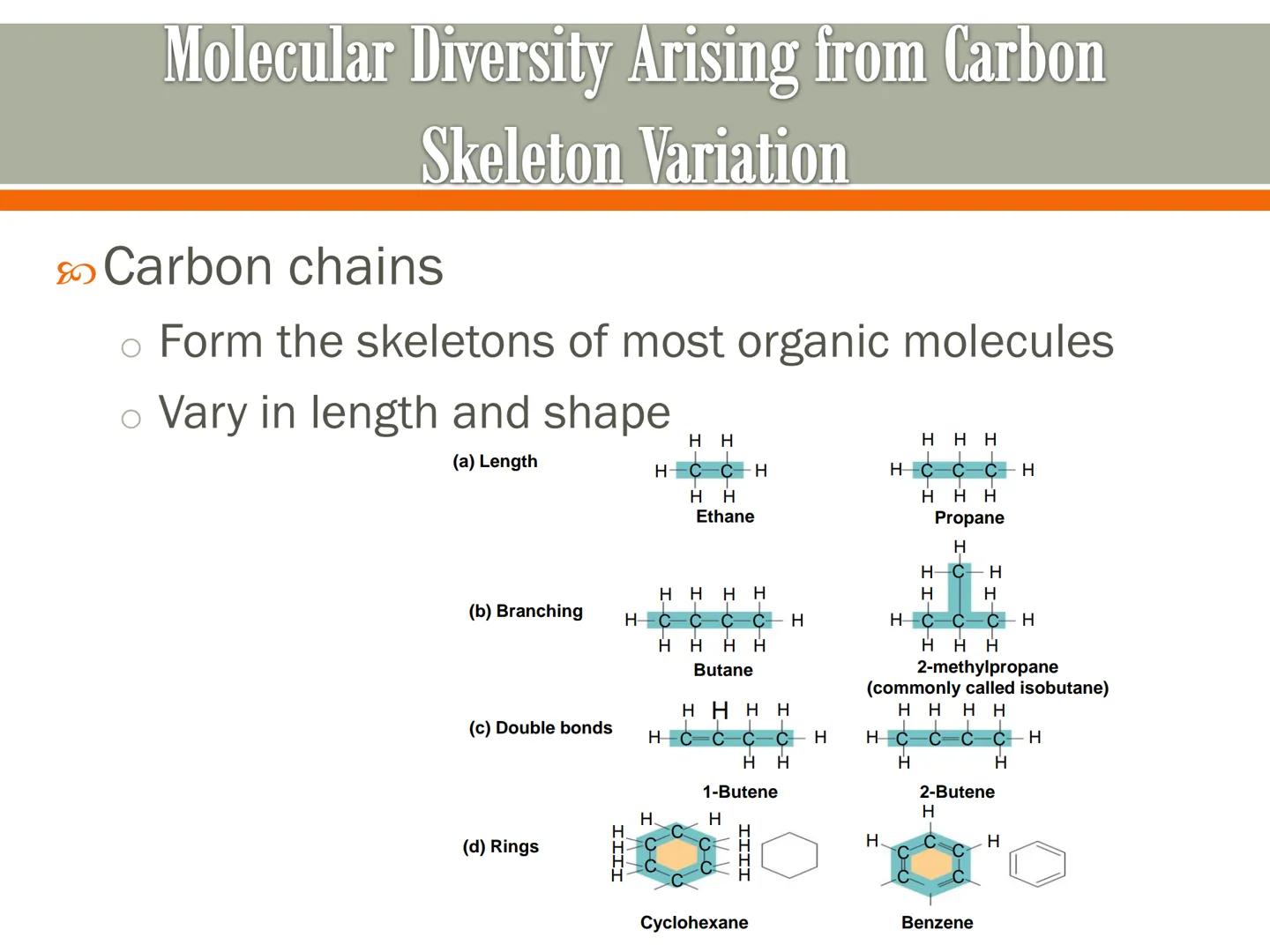
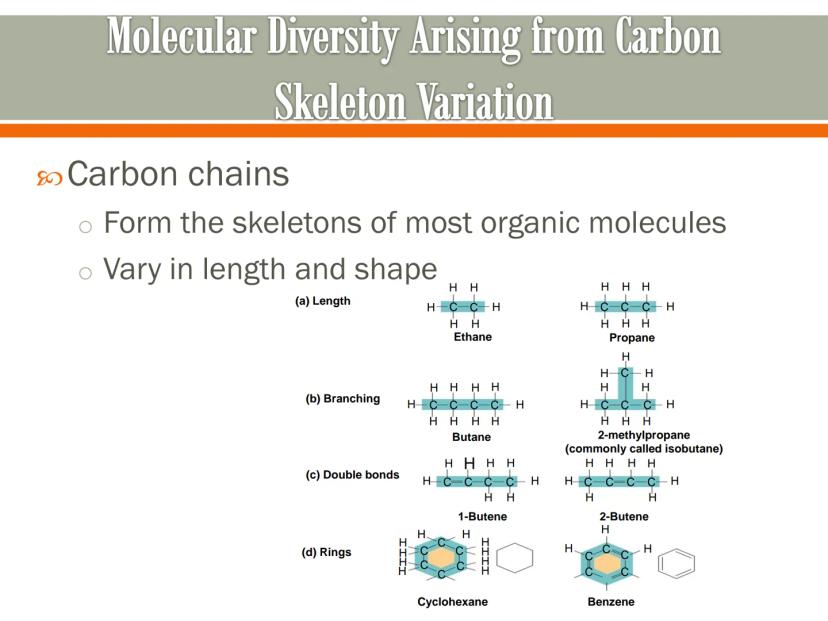
Carbon chains are the backbones of most organic molecules, and they can vary dramatically in both length and shape. This variation is what creates molecular diversity.
Carbon skeletons can differ in length - from ethane (2 carbons) to propane (3 carbons) to butane (4 carbons) and beyond. The longer the chain, the more complex the molecule can become.
These carbon backbones can also branch out, form rings, or include double bonds. For instance, butane and 2-methylpropane (isobutane) both have four carbon atoms, but their different arrangements give them different properties.
Real-World Connection: The difference between ordinary table sugar and starch is primarily in the length and arrangement of their carbon skeletons - small changes in carbon structure create big differences in function!
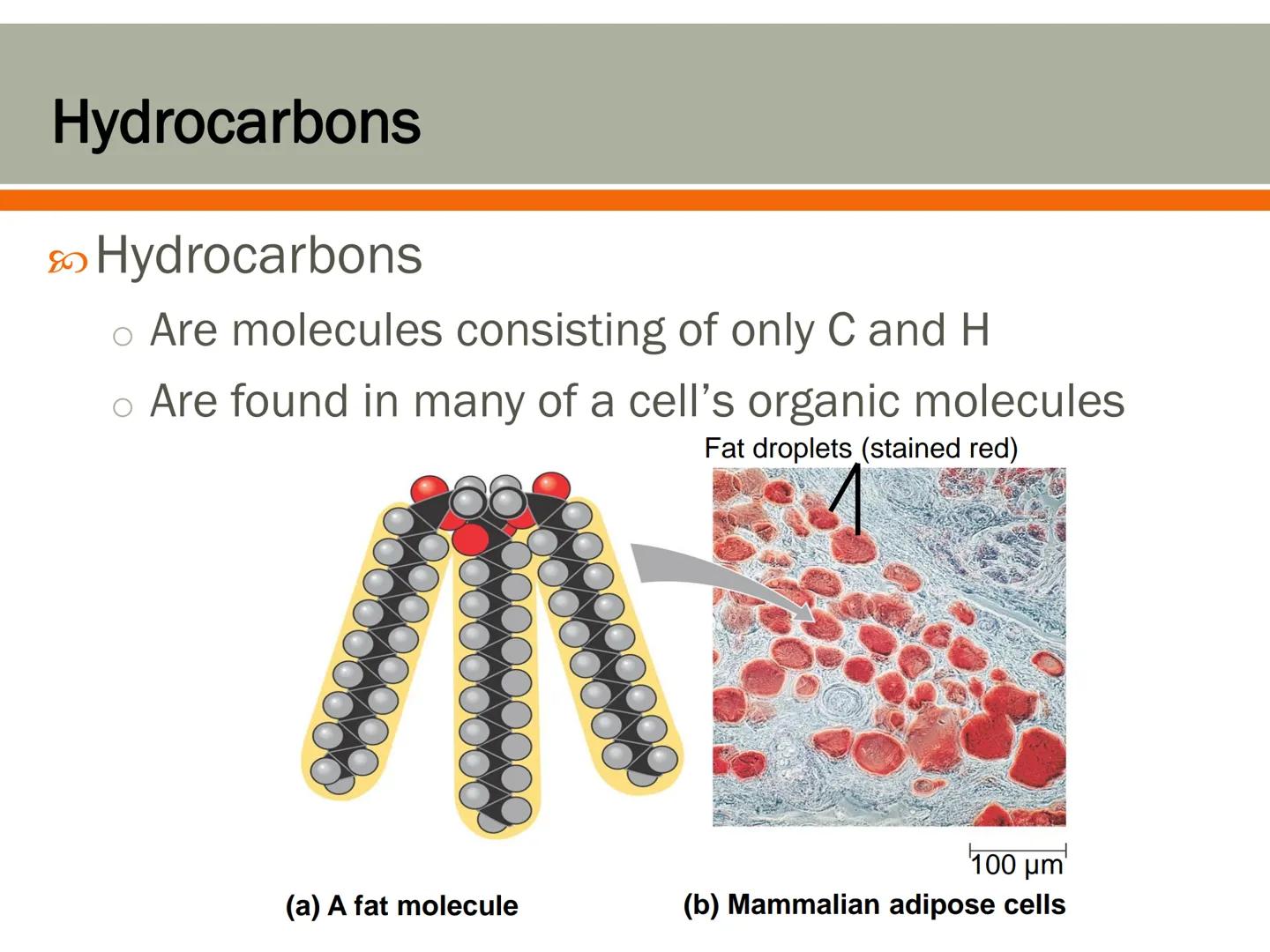
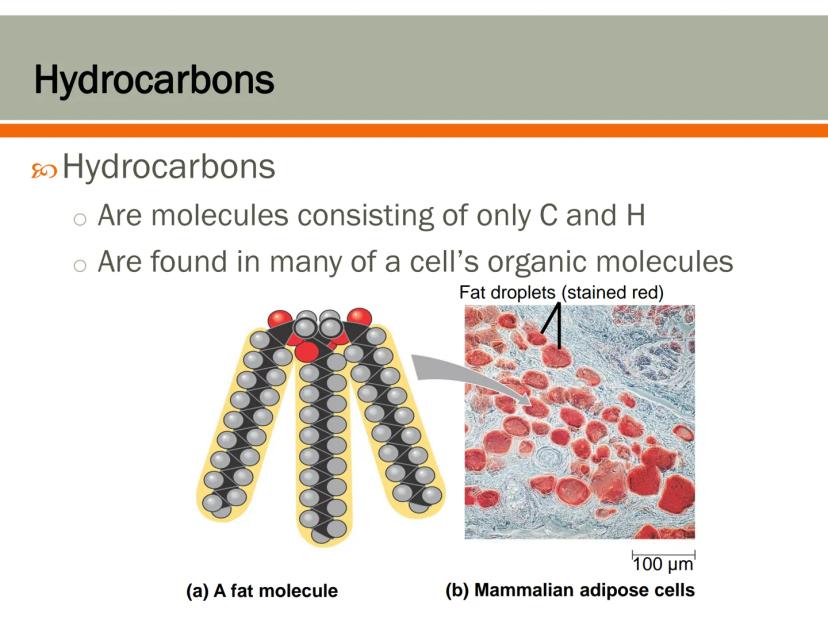
Hydrocarbons are molecules that contain only carbon and hydrogen atoms. They form the basic building blocks for many biological molecules, especially fats.
Your body stores energy in fat molecules, which contain large hydrocarbon sections. These hydrocarbon regions are hydrophobic , which is why oil and water don't mix.
Fat cells (adipose tissue) in mammals contain large droplets of these hydrocarbon-rich molecules. These cells are specially designed to store energy in a concentrated form, which is why fat is such an efficient way for your body to save energy for later use.
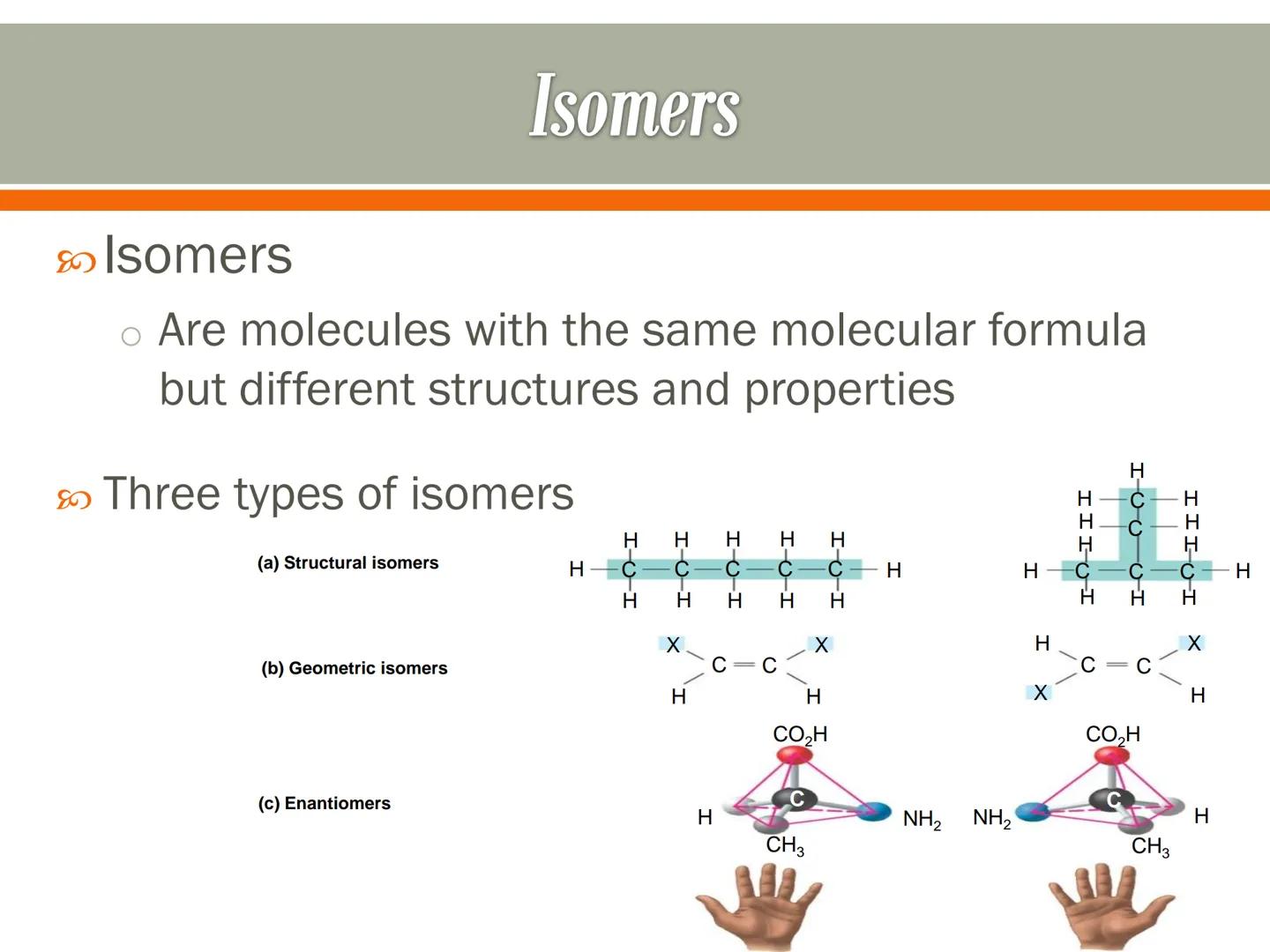
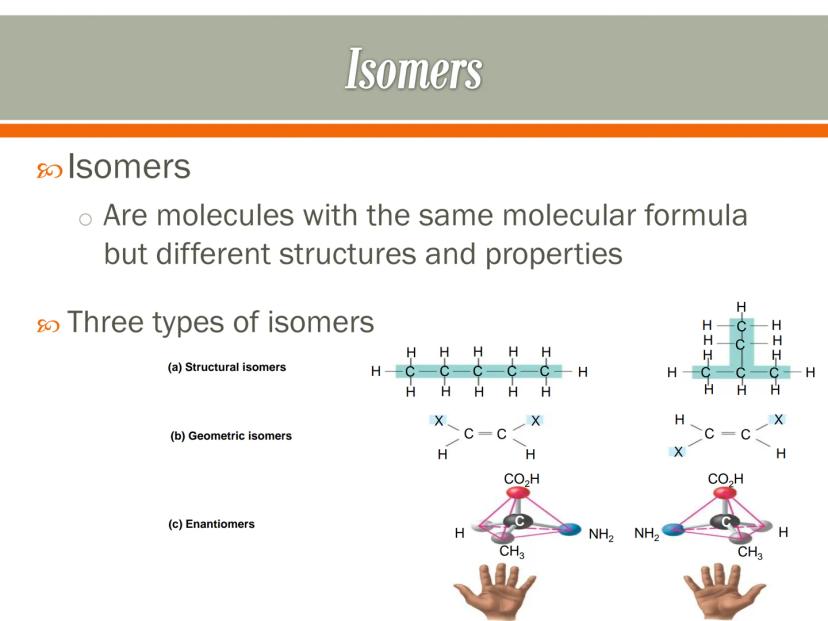
Isomers are molecules that have the same molecular formula but different structures and properties. It's like having the same puzzle pieces arranged in different patterns to create distinct pictures.
There are three main types of isomers. Structural isomers have atoms connected in different arrangements - like rearranging the rooms in a house. Geometric isomers have the same connections but different spatial arrangements - like mirror images that can't be superimposed.
Enantiomers are mirror images of each other that cannot be superimposed - like your left and right hands. They have identical chemical properties but can interact differently with other molecules in biological systems.
Amazing Fact: Your body can often tell the difference between enantiomers! One form might taste sweet while its mirror image is tasteless, or one might be medicine while the other is ineffective or even harmful.
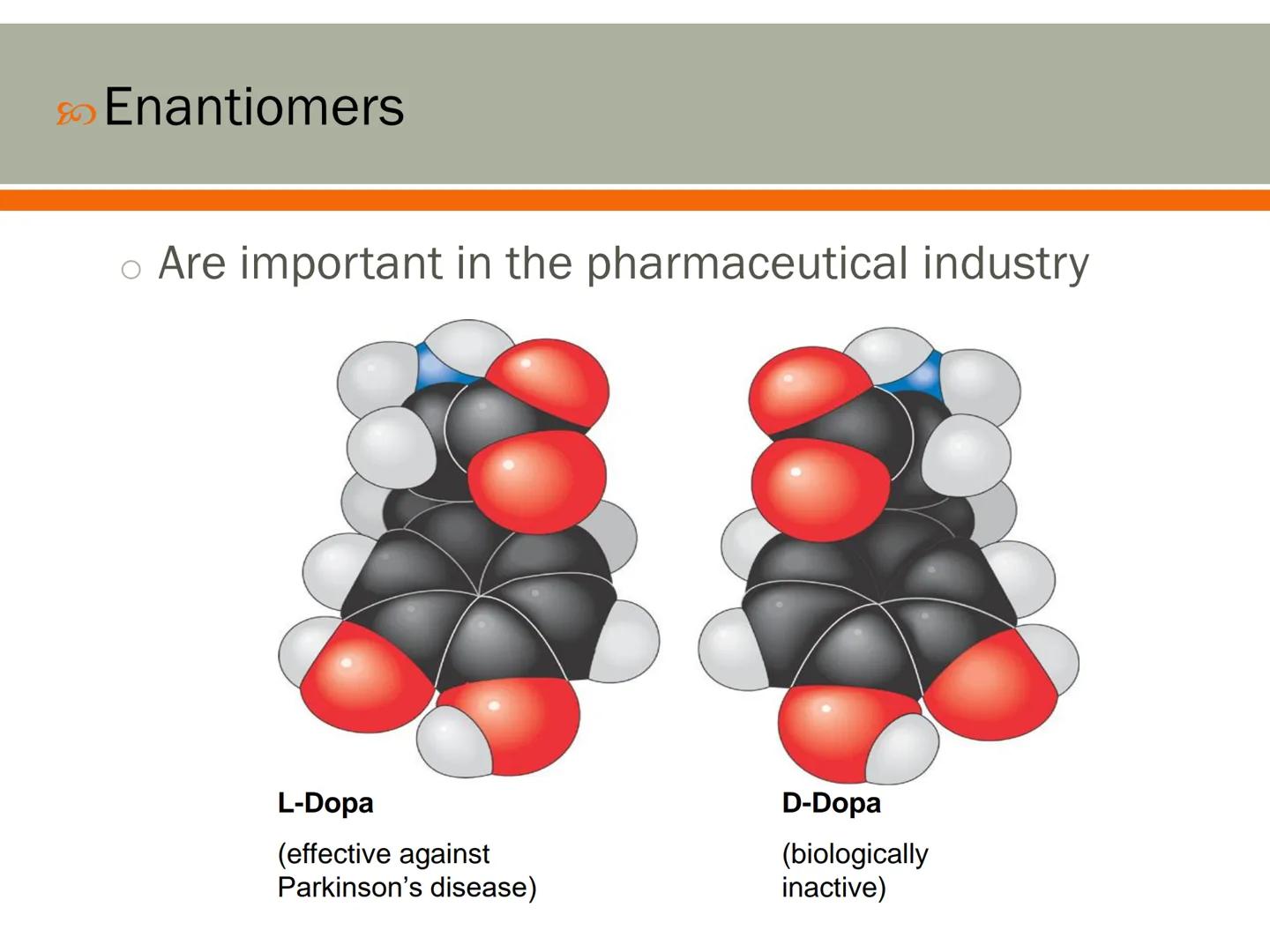
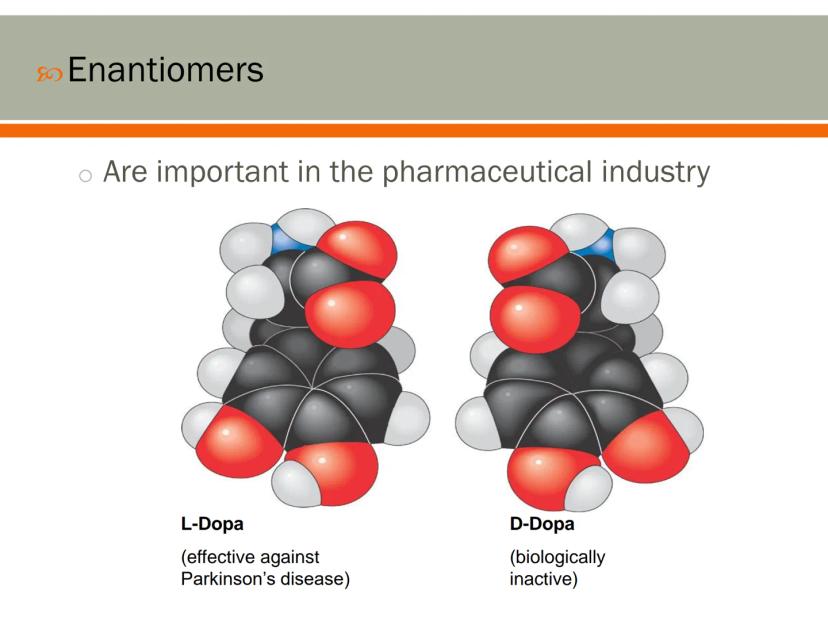
Enantiomers play a crucial role in pharmaceuticals because our bodies can respond very differently to each version of a molecule. This has huge implications for drug development and effectiveness.
A perfect example is L-Dopa and D-Dopa. Though they're mirror images with the same atoms, L-Dopa effectively treats Parkinson's disease by replacing missing dopamine in the brain, while D-Dopa has no biological effect at all.
Many drugs today are carefully manufactured to contain only the effective enantiomer. This precision helps reduce side effects and increase the medicine's potency.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Jasy Rodriguez
@jasyrodriguez_fpij
Carbon is the backbone of all life on Earth. Its unique chemical properties allow it to form an incredible variety of molecules that make up living organisms. Understanding carbon chemistry helps us grasp how simple atoms can create the complex... Show more

Access to all documents
Improve your grades
Join milions of students
Carbon is truly remarkable - it forms the foundation for all biological molecules in every living thing on our planet. This versatile element creates an astonishing range of compounds that give life its incredible diversity.
Think of carbon as the alphabet of life - just as 26 letters can form countless words, carbon atoms can arrange themselves into millions of different molecules with unique functions.
Fun Fact: The study of carbon compounds is called organic chemistry because these molecules were once thought to exist only within living organisms!

Access to all documents
Improve your grades
Join milions of students
Ever wonder what you're actually made of? At the molecular level, you're primarily carbon-based! All living organisms are built from chemical compounds that center around carbon atoms.
Organic chemistry specifically studies carbon compounds. This field explores how carbon's unique properties allow it to create the building blocks of life - from simple sugars to complex proteins that make you who you are.
When scientists analyze living tissues, they find carbon everywhere. It's in your DNA, proteins, fats, and even the molecules that provide energy to your cells.

Access to all documents
Improve your grades
Join milions of students
People once believed that organic compounds could only be created inside living things - an idea called vitalism. They thought living organisms had a special "vital force" that non-living things lacked.
This idea was challenged in a groundbreaking 1953 experiment by Stanley Miller. He recreated what scientists believed were Earth's early conditions - water vapor, hydrogen, ammonia, and methane - and added electrical sparks to simulate lightning.
The results were amazing! Miller's experiment produced several organic compounds essential for life, including amino acids (the building blocks of proteins). This suggested that the molecules needed for life could form naturally without any "vital force."
Mind-Blowing: Miller's experiment showed that the basic ingredients for life could have formed on early Earth through natural chemical reactions, possibly setting the stage for life's beginnings!

Access to all documents
Improve your grades
Join milions of students
Carbon's superpower is its ability to form bonds with up to four other atoms simultaneously. This makes it incredibly versatile compared to most other elements.
Why can carbon form four bonds? It has four valence electrons (electrons in its outer shell) that can be shared with other atoms to form stable covalent bonds.
This four-bond capability is like having four arms to connect with different partners. Carbon can bond with various elements including hydrogen, oxygen, nitrogen, and even other carbon atoms. This flexibility allows carbon to create chains, rings, and branches - the essential frameworks for biological molecules.

Access to all documents
Improve your grades
Join milions of students
Carbon's bonding abilities allow it to form incredibly diverse molecular structures. These carbon skeletons serve as frameworks for the molecules that make up your body.
Simple carbon compounds like methane (CH₄) have just one carbon atom bonded to four hydrogen atoms. More complex molecules like ethane (C₂H₆) feature carbon atoms bonded to each other, creating chains.
Carbon can also form double bonds, as seen in ethene (C₂H₄). These double bonds create different shapes and properties in molecules, adding to the diversity of possible structures.
Chemistry Hack: When visualizing molecules, different models help show different aspects - structural formulas show the bonds, while space-filling models show the actual shape and size!

Access to all documents
Improve your grades
Join milions of students
Carbon's electron configuration makes it extremely compatible with different elements. This compatibility is key to creating the diverse molecules found in living organisms.
Carbon readily forms bonds with hydrogen (which forms 1 bond), oxygen (which forms 2 bonds), and nitrogen (which forms 3 bonds). This allows carbon to be the central connecting element in most biological molecules.
Think of carbon as the ultimate team player in the molecular world. It can connect with almost any other element, creating countless combinations that serve different functions in living systems.

Access to all documents
Improve your grades
Join milions of students
Carbon chains are the backbones of most organic molecules, and they can vary dramatically in both length and shape. This variation is what creates molecular diversity.
Carbon skeletons can differ in length - from ethane (2 carbons) to propane (3 carbons) to butane (4 carbons) and beyond. The longer the chain, the more complex the molecule can become.
These carbon backbones can also branch out, form rings, or include double bonds. For instance, butane and 2-methylpropane (isobutane) both have four carbon atoms, but their different arrangements give them different properties.
Real-World Connection: The difference between ordinary table sugar and starch is primarily in the length and arrangement of their carbon skeletons - small changes in carbon structure create big differences in function!

Access to all documents
Improve your grades
Join milions of students
Hydrocarbons are molecules that contain only carbon and hydrogen atoms. They form the basic building blocks for many biological molecules, especially fats.
Your body stores energy in fat molecules, which contain large hydrocarbon sections. These hydrocarbon regions are hydrophobic , which is why oil and water don't mix.
Fat cells (adipose tissue) in mammals contain large droplets of these hydrocarbon-rich molecules. These cells are specially designed to store energy in a concentrated form, which is why fat is such an efficient way for your body to save energy for later use.

Access to all documents
Improve your grades
Join milions of students
Isomers are molecules that have the same molecular formula but different structures and properties. It's like having the same puzzle pieces arranged in different patterns to create distinct pictures.
There are three main types of isomers. Structural isomers have atoms connected in different arrangements - like rearranging the rooms in a house. Geometric isomers have the same connections but different spatial arrangements - like mirror images that can't be superimposed.
Enantiomers are mirror images of each other that cannot be superimposed - like your left and right hands. They have identical chemical properties but can interact differently with other molecules in biological systems.
Amazing Fact: Your body can often tell the difference between enantiomers! One form might taste sweet while its mirror image is tasteless, or one might be medicine while the other is ineffective or even harmful.

Access to all documents
Improve your grades
Join milions of students
Enantiomers play a crucial role in pharmaceuticals because our bodies can respond very differently to each version of a molecule. This has huge implications for drug development and effectiveness.
A perfect example is L-Dopa and D-Dopa. Though they're mirror images with the same atoms, L-Dopa effectively treats Parkinson's disease by replacing missing dopamine in the brain, while D-Dopa has no biological effect at all.
Many drugs today are carefully manufactured to contain only the effective enantiomer. This precision helps reduce side effects and increase the medicine's potency.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
3
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
Cell metabolism is a network of biochemical reactions transforming metabolites to fulfill biological functions.
monosaccharides, disaccharides, polysaccharides, glycosidic linkage, phospholipids, ester linkage
Explore the essential characteristics of living organisms, including movement, respiration, sensitivity, growth, reproduction, excretion, and nutrition. This summary provides key definitions and a mnemonic to help you remember these vital biological processes. Ideal for biology students seeking to understand the fundamental traits that define life.
Notes and key concepts on Water and Life
8.1-8.6
Reactions of photosynthesis
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user