States of Matter
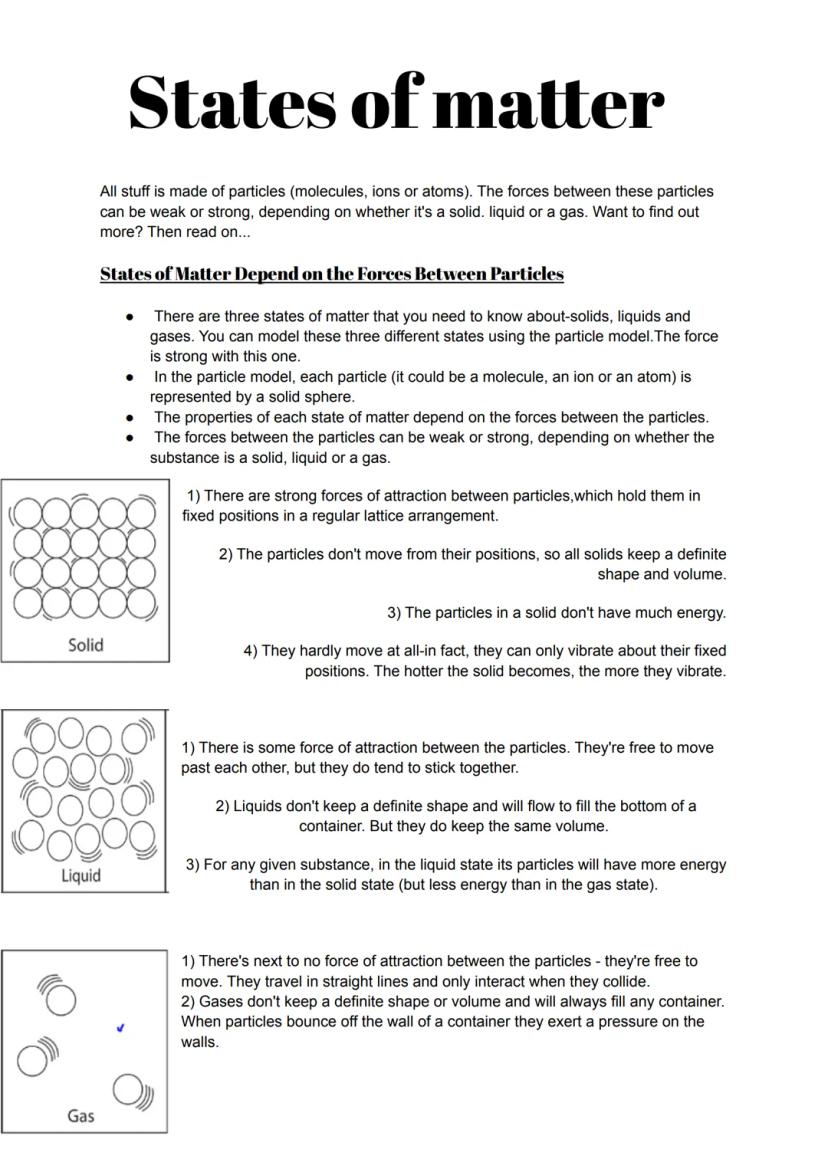
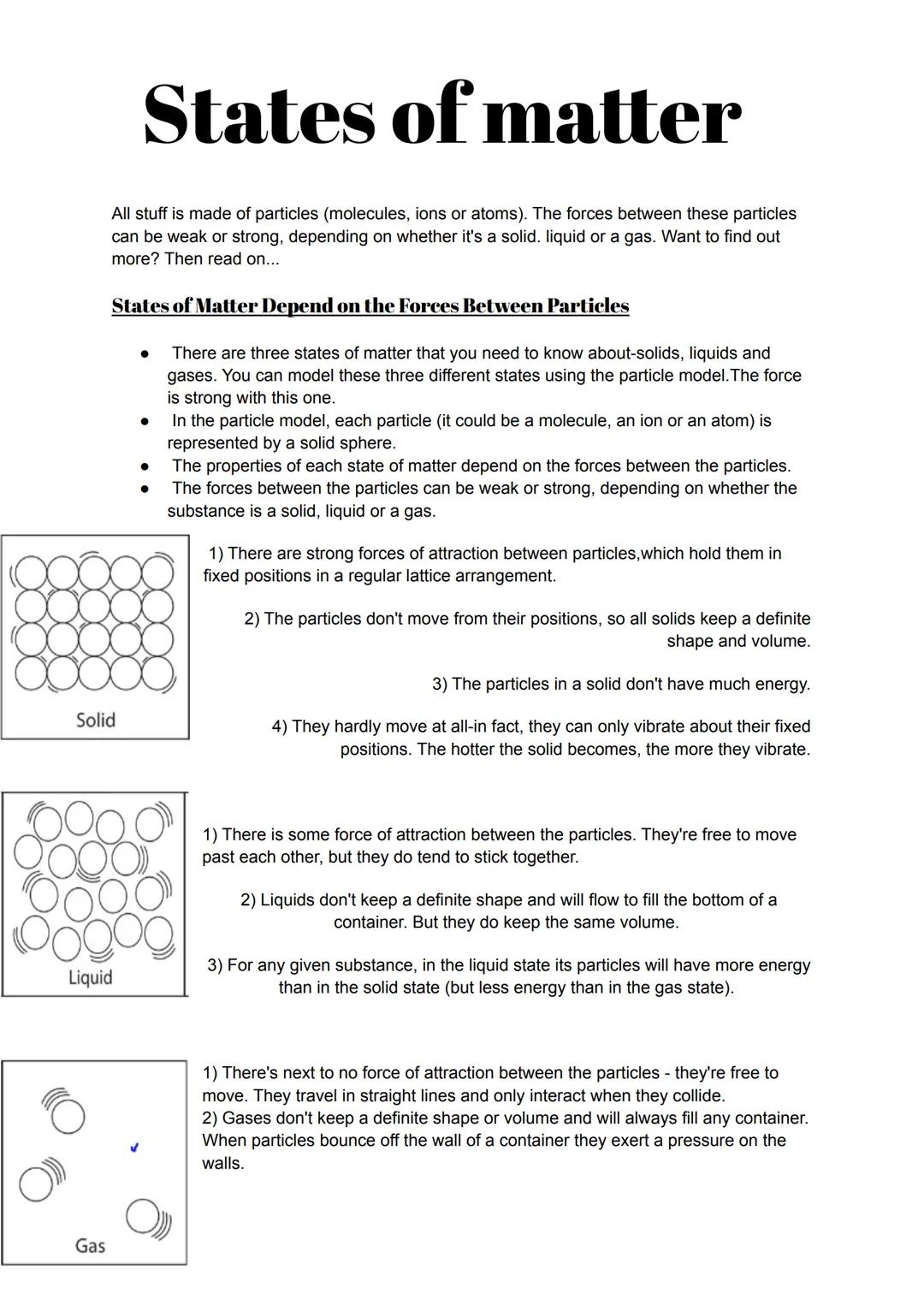
Every single thing around you is made of tiny particles, and how these particles behave determines whether something is solid, liquid, or gas. The particle model helps us picture this using solid spheres to represent each particle.
In solids, particles are locked in place by strong forces, creating rigid structures. They can only vibrate - which is why your desk doesn't suddenly become wobbly! Liquids have weaker forces between particles, letting them slide past each other whilst staying together. This is why water takes the shape of its container but doesn't fly apart.
Gases have virtually no forces holding particles together. The particles zoom around in straight lines, bouncing off walls and creating pressure. This explains why you can compress a syringe filled with air but not one filled with water.
Remember: More energy means particles move more - that's why ice melts when heated and water boils to become steam.