Acid-base equilibria are central to understanding chemical reactions in both... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Knowunity AI
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
232
•
Feb 9, 2026
•
Acid-base equilibria are central to understanding chemical reactions in both... Show more





Acids and bases are fundamental chemical substances that interact through proton transfer. Their reactions create equilibria that follow predictable patterns based on their strengths.
When studying acid-base chemistry, we need to understand several key definitions. An acid can donate protons, while a base accepts protons. When these substances interact, they form products called salts plus water in a process called neutralization.
Strong acids and bases dissociate completely in water, whereas weak acids and bases only partially dissociate. This difference in behavior creates varying pH levels that we can measure and predict mathematically.
Remember: Water itself can act as both an acid and a base - this dual nature makes it amphoteric and is essential to understanding acid-base chemistry!

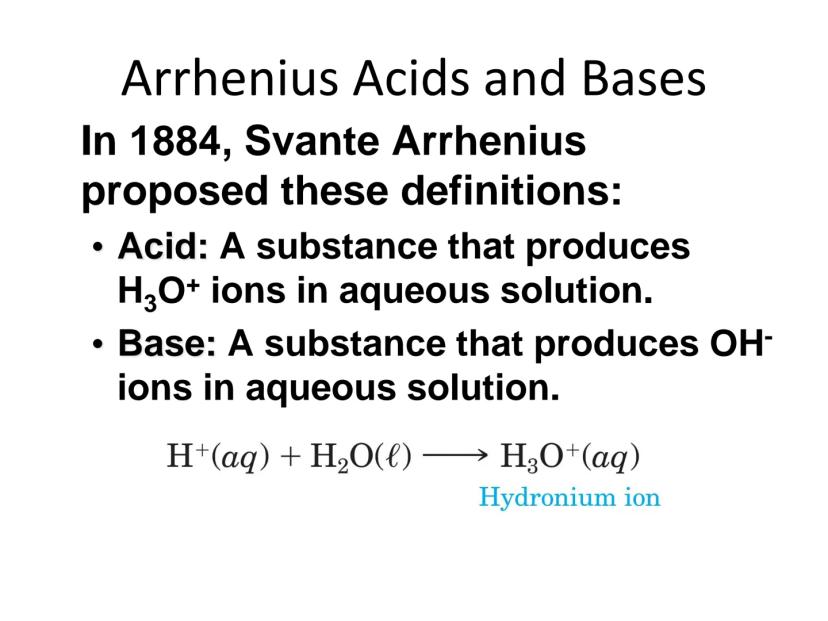
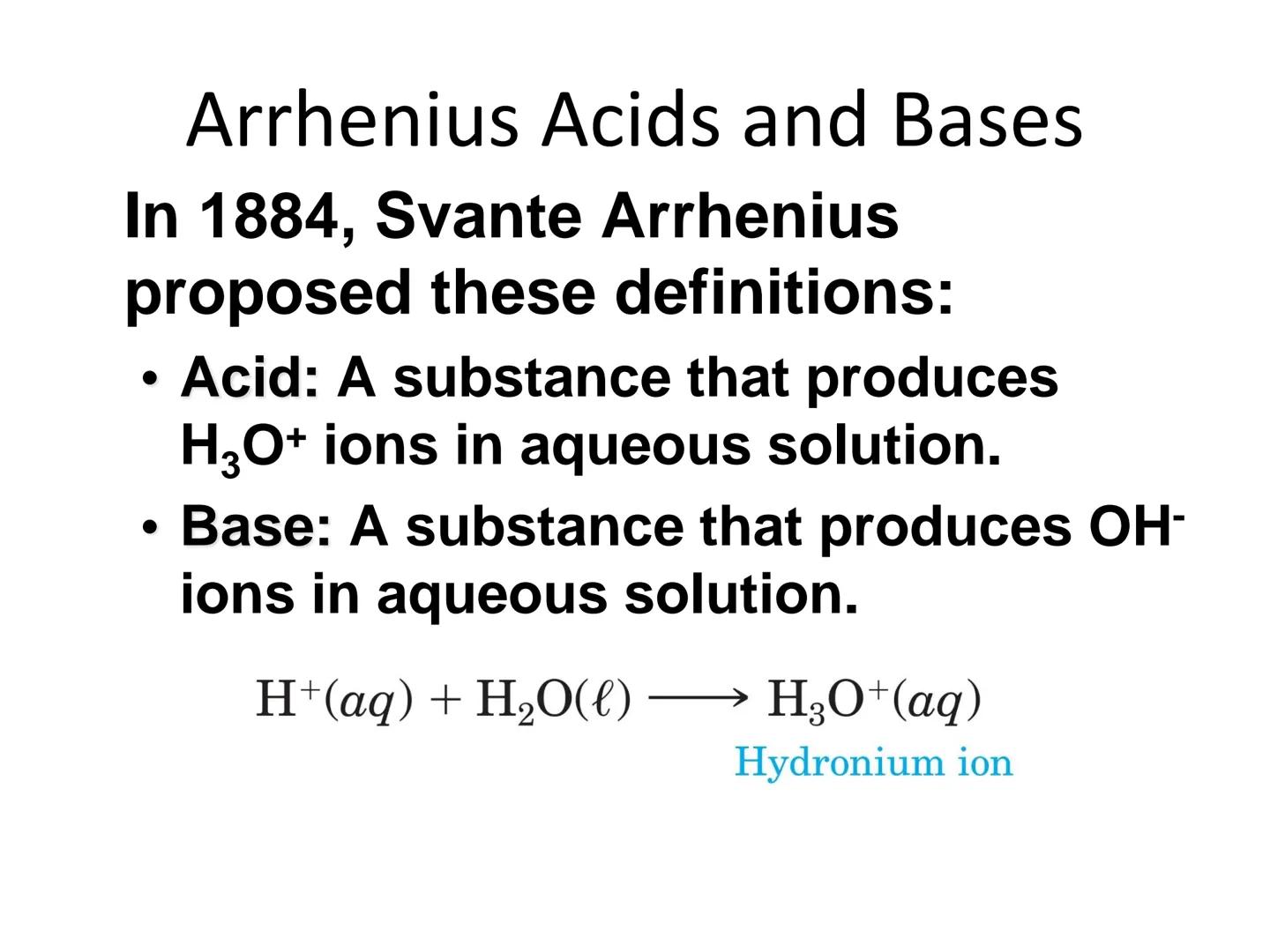
In 1884, Svante Arrhenius provided one of the earliest modern definitions of acids and bases. According to his theory, an acid is a substance that produces hydronium ions (H₃O⁺) in water, while a base produces hydroxide ions (OH⁻) in water.
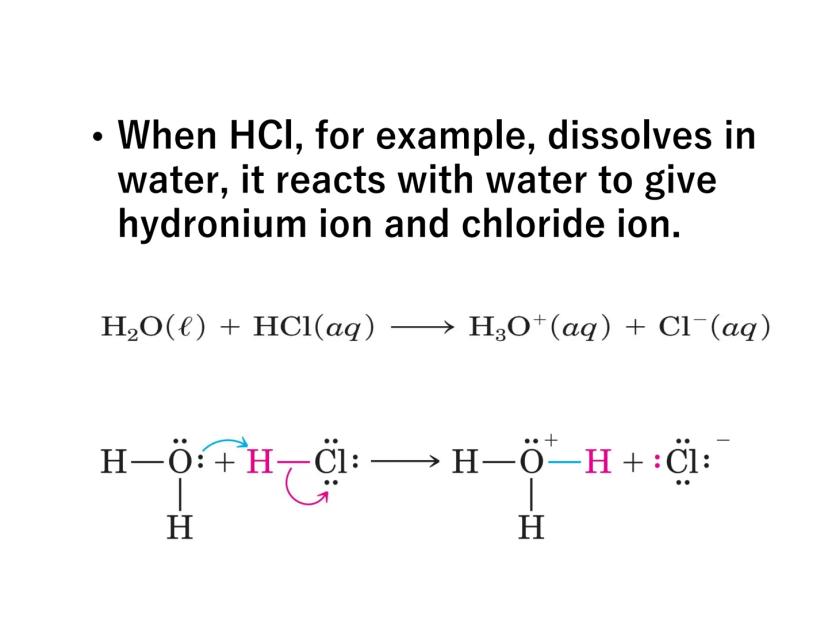
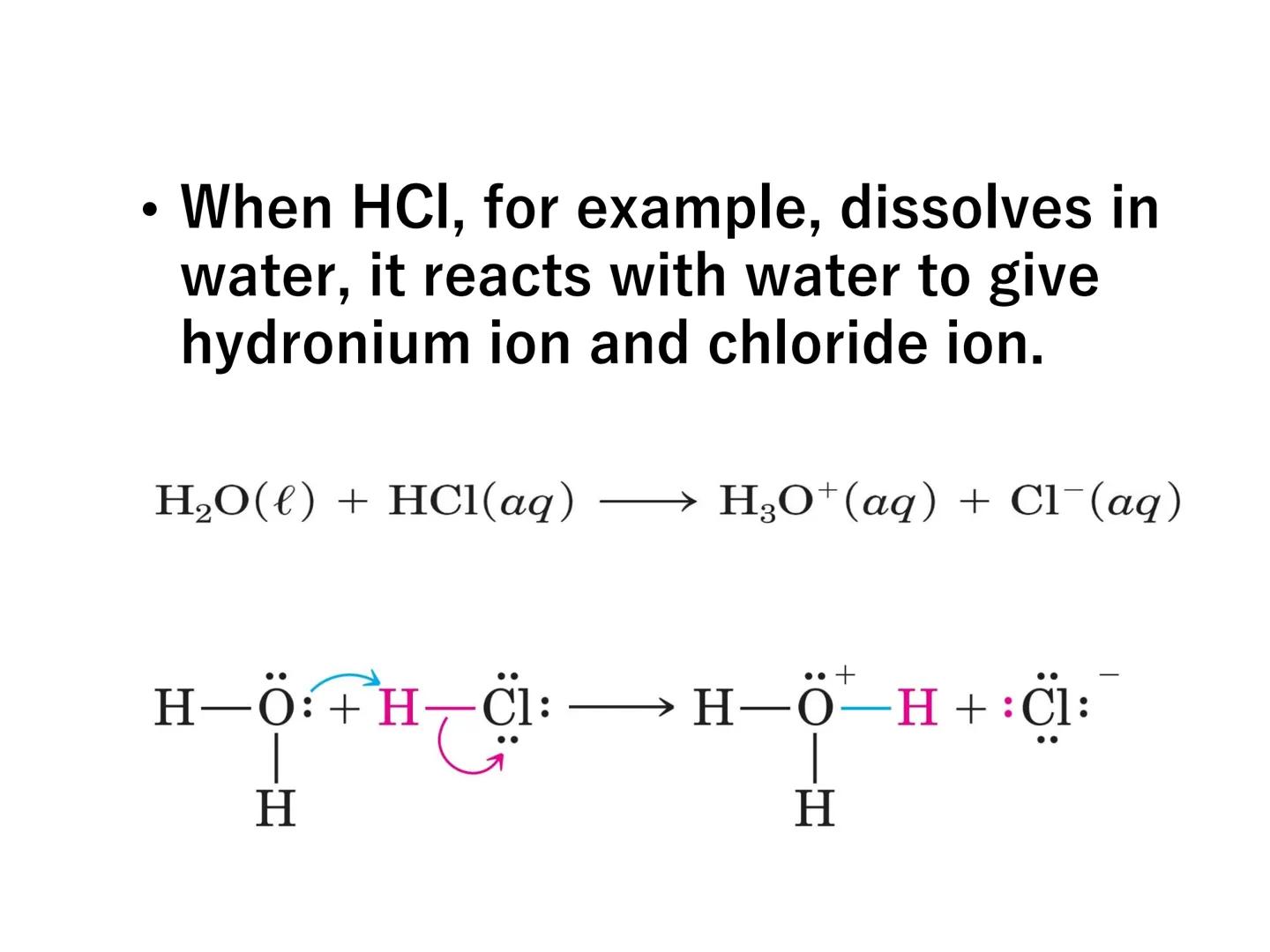
When an acid like HCl dissolves in water, it reacts to form hydronium ions:
H₂O(l) + HCl(aq) → H₃O⁺(aq) + Cl⁻(aq)
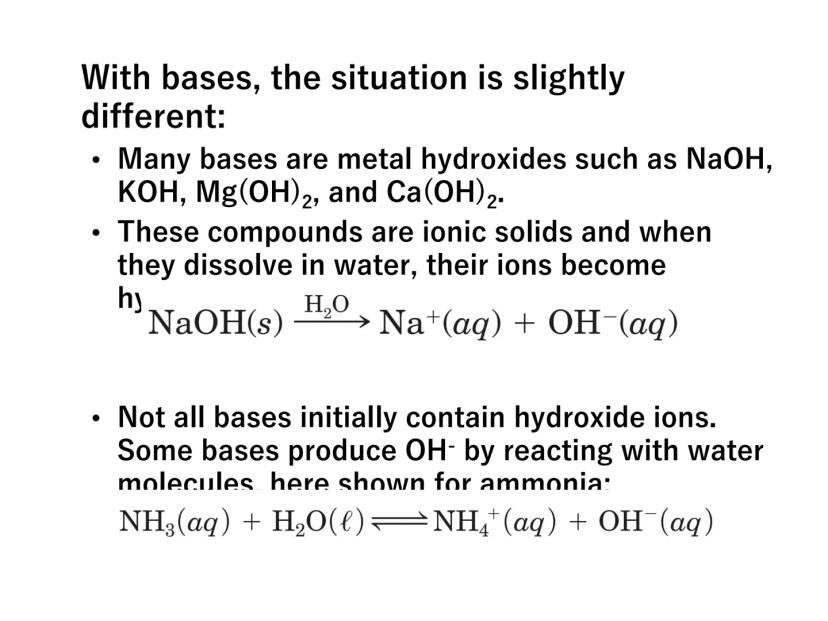
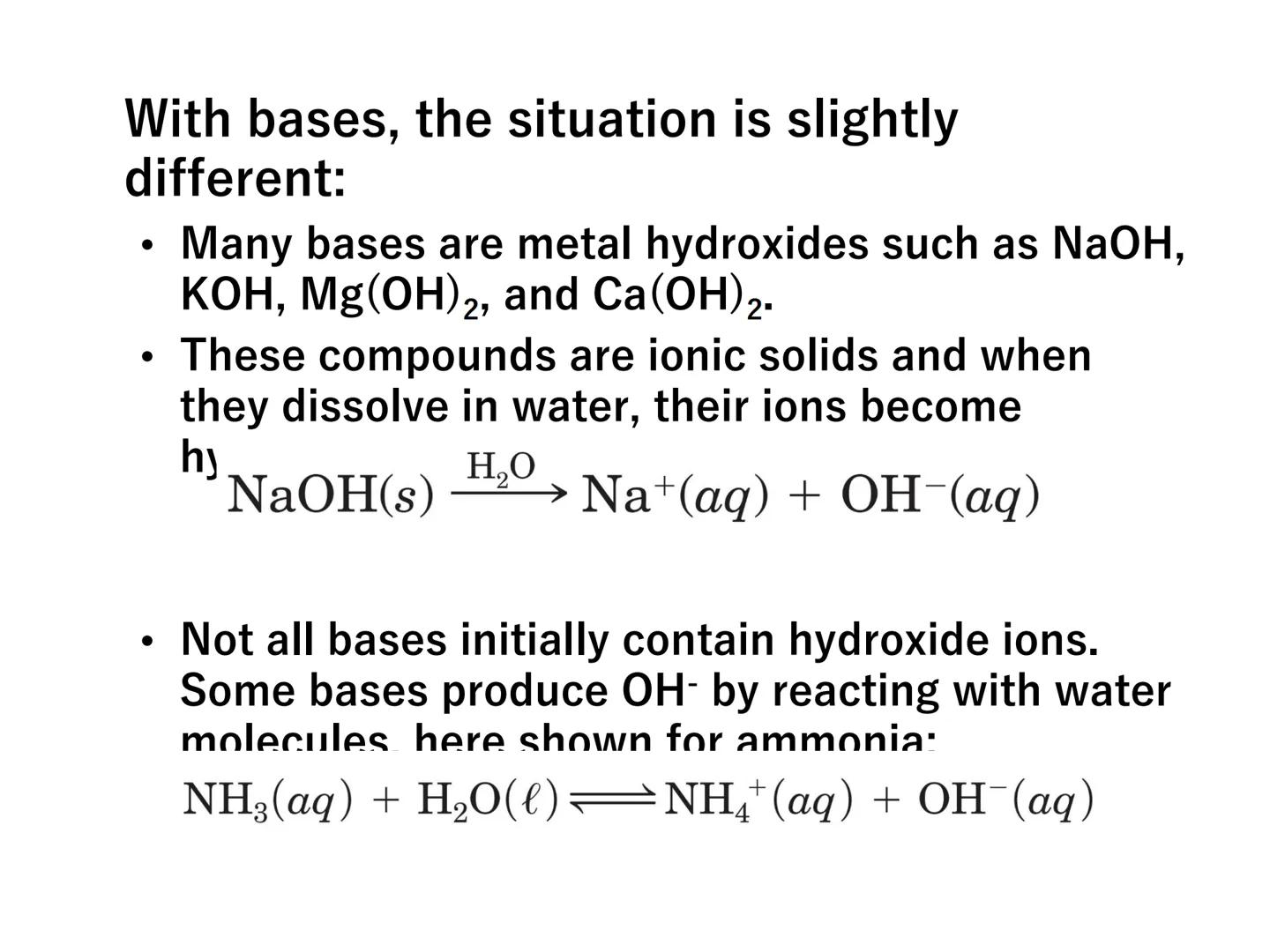
Bases work slightly differently. Many bases are metal hydroxides like NaOH or KOH that directly release OH⁻ ions when dissolved:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
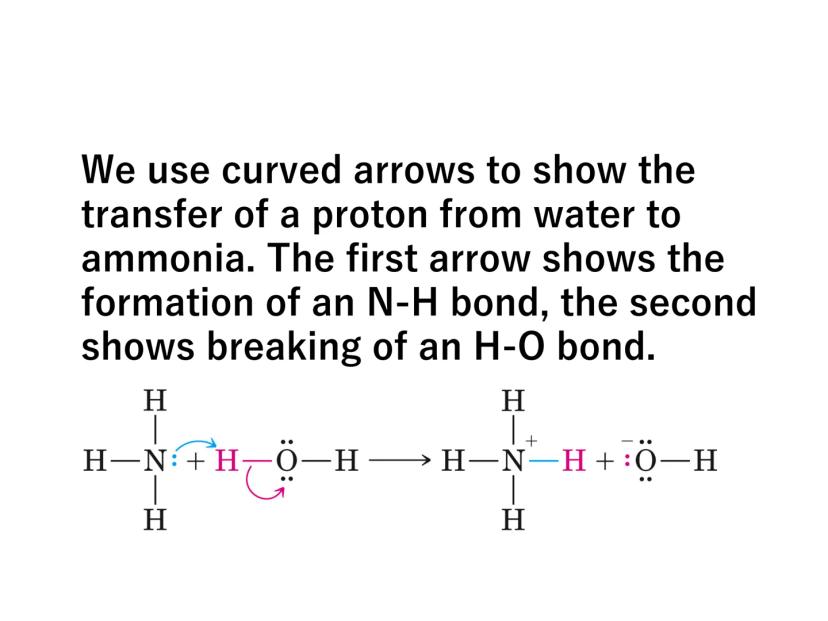
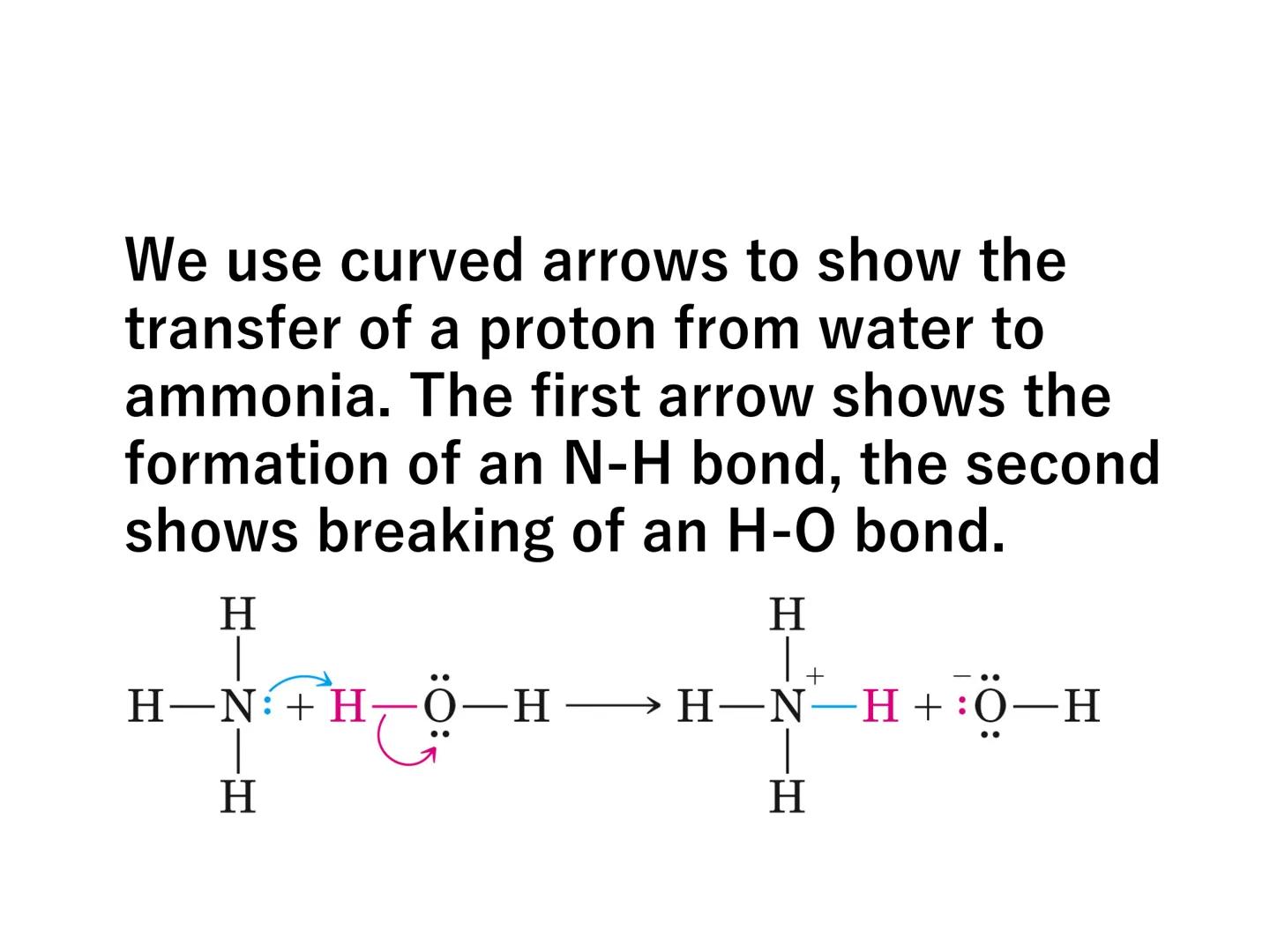
Some bases like ammonia (NH₃) don't initially contain hydroxide ions but produce them by reacting with water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Chemistry in Action: When ammonia reacts with water, a proton transfers from water to ammonia - this is shown using curved arrows in reaction mechanisms to track electron movement!

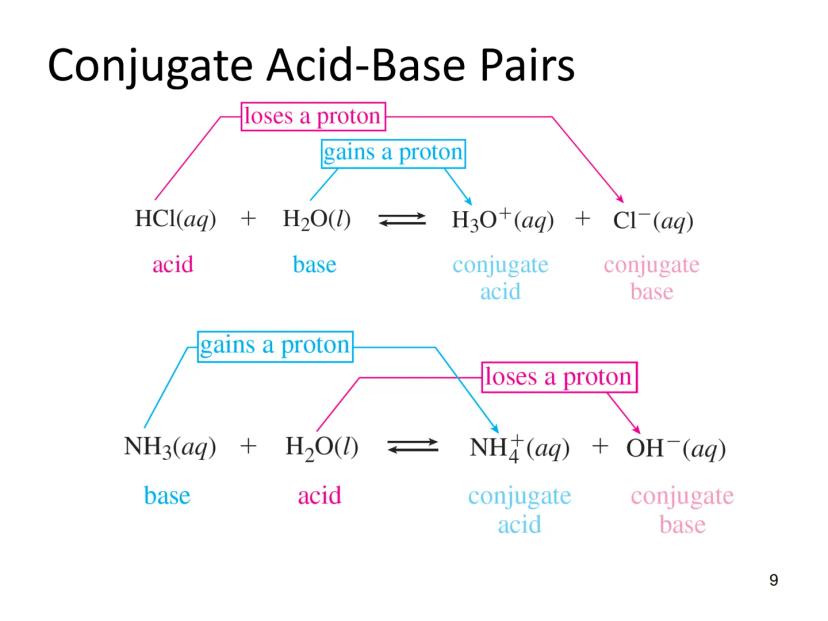
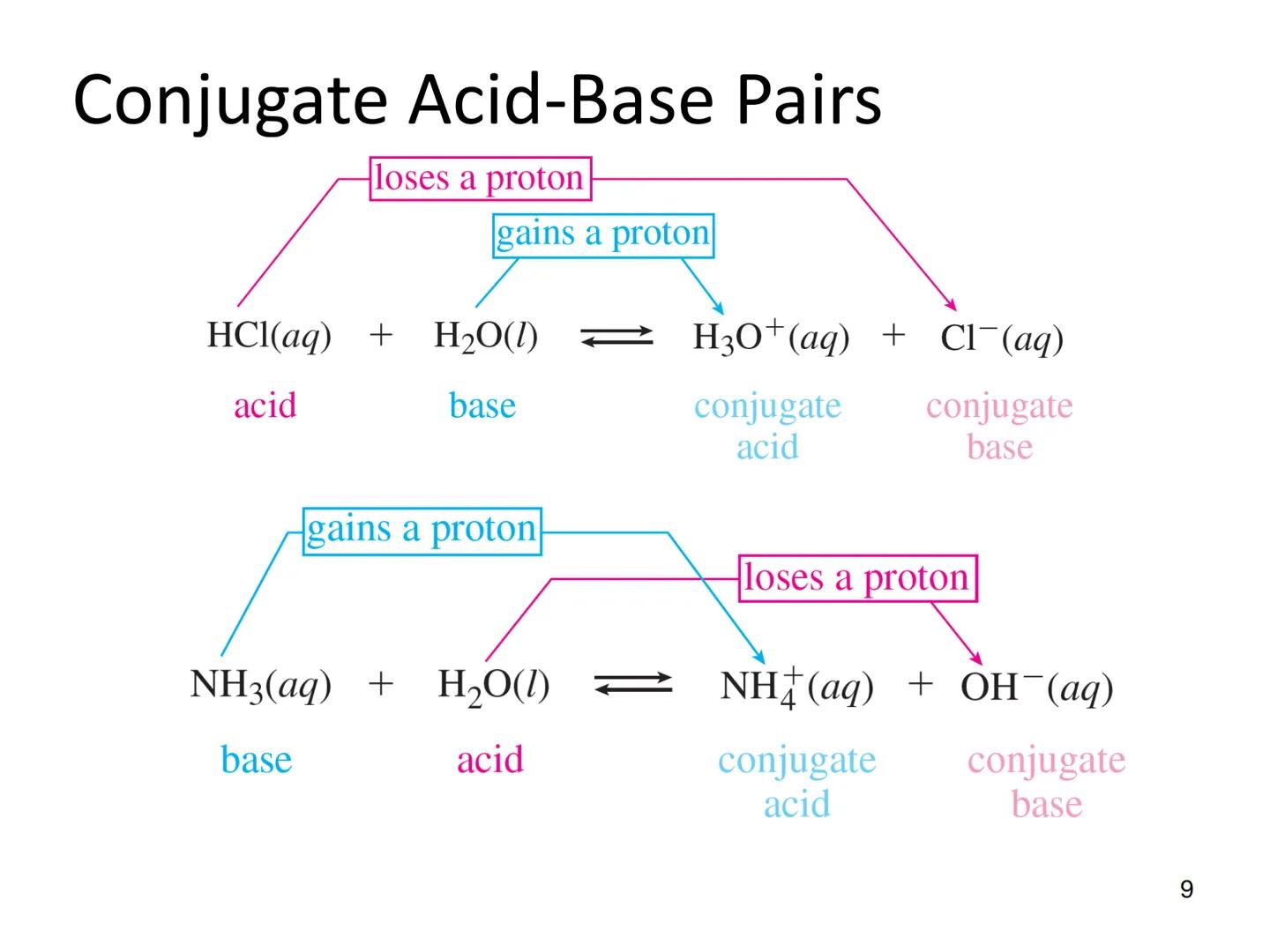
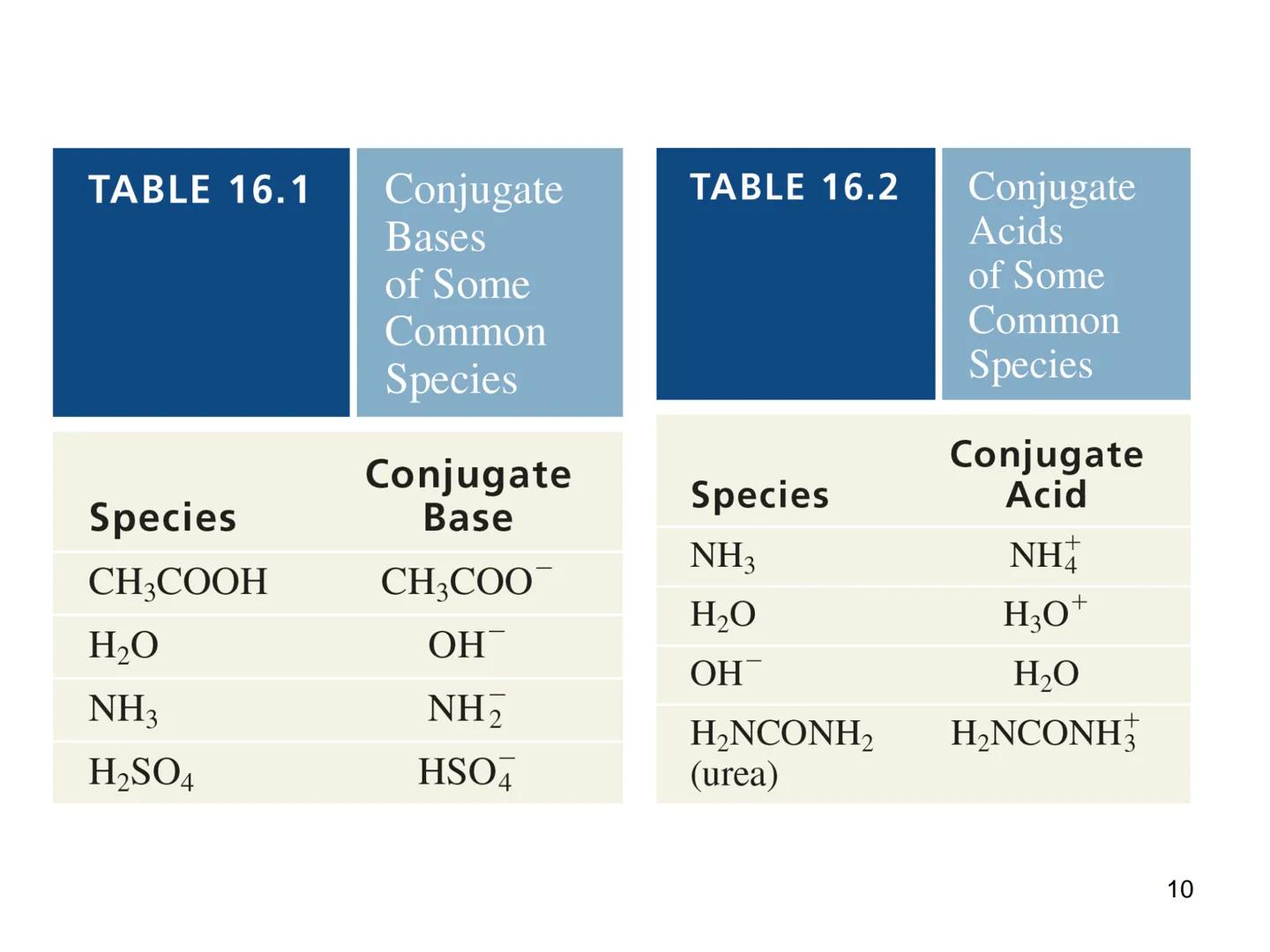
The Brønsted-Lowry definition expands our understanding of acids and bases beyond the Arrhenius theory. In this model, an acid is a proton donor, and a base is a proton acceptor.
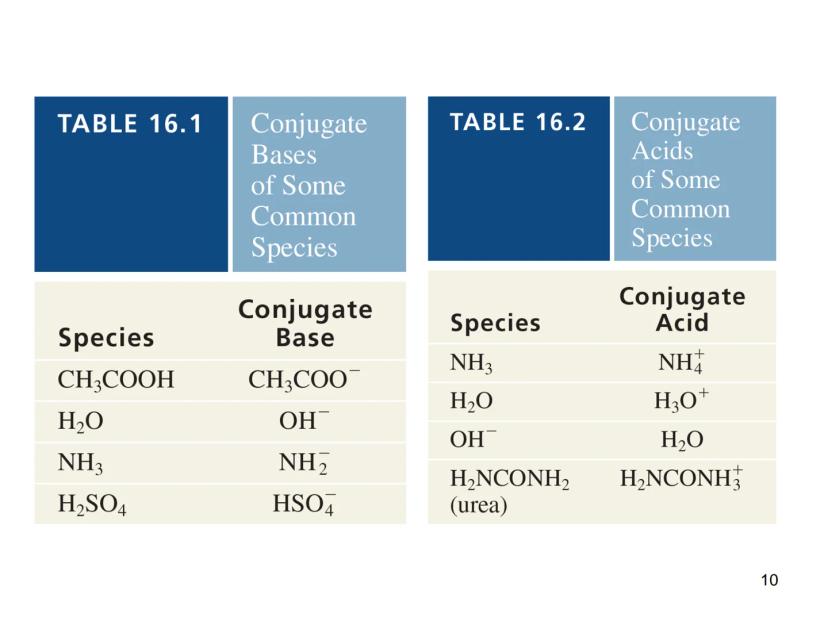
This definition introduces the important concept of conjugate pairs. When an acid donates a proton, what remains is called the conjugate base. Similarly, when a base accepts a proton, it forms a conjugate acid.
For example, in the reaction:
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
HCl is the acid, H₂O is the base, H₃O⁺ is the conjugate acid, and Cl⁻ is the conjugate base.
In another example:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
NH₃ acts as the base, H₂O as the acid, NH₄⁺ is the conjugate acid, and OH⁻ is the conjugate base.
Quick Tip: To identify conjugate pairs, look for species that differ by just one H⁺. For example, NH₃/NH₄⁺ and H₂O/OH⁻ are conjugate pairs.
The Lewis definition provides the most general way to understand acids and bases. A Lewis base is a substance that can donate a pair of electrons, while a Lewis acid accepts a pair of electrons.
This definition broadens our understanding beyond just proton transfer. A Lewis acid-base reaction involves the formation of a coordinate covalent bond, where both electrons in the new bond come from the same atom.
For example, when boron trifluoride (BF₃) reacts with ammonia (NH₃):
F₃B + :NH₃ → F₃B-NH₃
The nitrogen atom in ammonia donates its lone pair of electrons to form a bond with boron.
This definition explains reactions that don't involve hydrogen ions at all, making it the most comprehensive acid-base theory. It can describe interactions between metal ions and ligands, which is crucial in understanding coordination chemistry.
Why This Matters: The Lewis definition explains reactions in organic chemistry, biochemistry, and materials science that wouldn't be considered acid-base reactions under earlier models!
Water is special because it can act as both an acid and a base - a property called amphoteric. It can donate protons (acting as an acid) or accept them (acting as a base) depending on what it's reacting with.
Water undergoes a process called autoionization:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equilibrium has a constant called Kw:
Kw = [H₃O⁺][OH⁻] = 1 × 10⁻¹⁴ at 25°C
In pure water, [H₃O⁺] = [OH⁻] = 1 × 10⁻⁷ M, which creates a perfect balance. This balance determines whether a solution is:
The relationship between these ions is always governed by Kw, meaning if one concentration changes, the other must change to maintain their product at 1 × 10⁻¹⁴.
Think About It: Even in highly acidic solutions, there are still some OH⁻ ions present, just at very low concentrations!
The pH scale provides a convenient way to express the acidity or basicity of a solution without writing out scientific notation. The pH is defined as:
pH = -log[H⁺] or pH = -log[H₃O⁺]
This logarithmic scale means that each unit change in pH represents a tenfold change in hydrogen ion concentration. For instance, a solution with pH 3 has ten times more hydrogen ions than a solution with pH 4.
On this scale at 25°C:
Common substances have characteristic pH values: stomach acid (~1), orange juice (~3.5), pure water (7), blood (~7.4), and household ammonia (~11.5).
We can also express hydroxide concentration using pOH:
pOH = -log[OH⁻]
At 25°C, pH and pOH are related by:
pH + pOH = 14
Real-World Application: Your body maintains blood pH between 7.35-7.45 - even small deviations can be life-threatening, showing how critical pH balance is to biological systems!
Strong acids and bases completely dissociate in water, meaning essentially 100% of their molecules break apart into ions. This dissociation is so complete that it's not considered an equilibrium process.
Common strong acids include:
Common strong bases include:
For strong acids, the hydronium ion concentration [H₃O⁺] equals the initial concentration of the acid. Similarly, for strong bases, the hydroxide concentration [OH⁻] equals the initial concentration multiplied by the number of hydroxide ions per formula unit.
For example, in a 0.057 M HBr solution:
HBr(aq) + H₂O(l) → H₃O⁺(aq) + Br⁻(aq)
[H₃O⁺] = 0.057 M, giving a pH of 1.24
Important: Remember that only a few acids and bases are strong - most are weak and follow equilibrium principles!

Weak acids only partially ionize in water, establishing an equilibrium between the acid and its ions. The extent of this ionization depends on both the acid's concentration and its acid ionization constant, Ka.
For a weak acid HA:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
The acid ionization constant is:
Ka = [H₃O⁺][A⁻]/[HA]
Common weak acids include acetic acid , formic acid , and hydrofluoric acid .
To calculate the pH of a weak acid solution, we set up an equilibrium table with:
If Ka is small compared to the initial concentration, we can use the approximation:
Ka ≈ x²/Ci
This approximation is valid if x < 5% of the initial acid concentration.
Problem-Solving Tip: When calculating weak acid pH, start by assuming x is small. If your answer shows x > 5% of the initial concentration, you'll need to solve the quadratic equation without the approximation!
Similar to weak acids, weak bases only partially ionize in water. Their ionization is characterized by the base ionization constant, Kb.
For a generic weak base B:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The base ionization constant is:
Kb = [BH⁺][OH⁻]/[B]
Common weak bases include ammonia , methylamine , and pyridine .
The approach for solving weak base problems mirrors that for weak acids, except that we solve for [OH⁻] rather than [H₃O⁺]. Once we know [OH⁻], we can calculate pOH and then pH.
For example, if a 0.50 M weak base solution has pH 9.59, we can determine that:
Real-Life Connection: Many medications and household products are weak bases. Understanding their chemistry helps pharmacists formulate drugs and chemists develop effective cleaning products!
There's an inverse relationship between the strength of an acid or base and its conjugate. A strong acid will have a weak conjugate base since it readily gives up its proton. Conversely, a weak acid will have a strong conjugate base that's eager to reclaim a proton.
For example:
This relationship can be quantified. For any conjugate acid-base pair, the product of their ionization constants equals the water ionization constant:
Ka × Kb = Kw = 1 × 10⁻¹⁴
This means if we know the Ka of an acid, we can calculate the Kb of its conjugate base:
Kb = Kw/Ka
For example, if benzoic acid has Ka = 6.5 × 10⁻⁵, then its conjugate base (benzoate ion) has Kb = 1.5 × 10⁻¹⁰.
Study Strategy: When you learn the strength of an acid, you automatically know something about its conjugate base. This pattern helps you predict chemical behavior without memorizing every value!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Acid-base equilibria are central to understanding chemical reactions in both laboratory and real-world settings. These reactions involve the transfer of protons (H⁺) between substances, creating a delicate balance that affects everything from our body's pH to the environment around us.... Show more

Access to all documents
Improve your grades
Join milions of students
Acids and bases are fundamental chemical substances that interact through proton transfer. Their reactions create equilibria that follow predictable patterns based on their strengths.
When studying acid-base chemistry, we need to understand several key definitions. An acid can donate protons, while a base accepts protons. When these substances interact, they form products called salts plus water in a process called neutralization.
Strong acids and bases dissociate completely in water, whereas weak acids and bases only partially dissociate. This difference in behavior creates varying pH levels that we can measure and predict mathematically.
Remember: Water itself can act as both an acid and a base - this dual nature makes it amphoteric and is essential to understanding acid-base chemistry!

Access to all documents
Improve your grades
Join milions of students
In 1884, Svante Arrhenius provided one of the earliest modern definitions of acids and bases. According to his theory, an acid is a substance that produces hydronium ions (H₃O⁺) in water, while a base produces hydroxide ions (OH⁻) in water.
When an acid like HCl dissolves in water, it reacts to form hydronium ions:
H₂O(l) + HCl(aq) → H₃O⁺(aq) + Cl⁻(aq)
Bases work slightly differently. Many bases are metal hydroxides like NaOH or KOH that directly release OH⁻ ions when dissolved:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
Some bases like ammonia (NH₃) don't initially contain hydroxide ions but produce them by reacting with water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Chemistry in Action: When ammonia reacts with water, a proton transfers from water to ammonia - this is shown using curved arrows in reaction mechanisms to track electron movement!

Access to all documents
Improve your grades
Join milions of students
The Brønsted-Lowry definition expands our understanding of acids and bases beyond the Arrhenius theory. In this model, an acid is a proton donor, and a base is a proton acceptor.
This definition introduces the important concept of conjugate pairs. When an acid donates a proton, what remains is called the conjugate base. Similarly, when a base accepts a proton, it forms a conjugate acid.
For example, in the reaction:
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
HCl is the acid, H₂O is the base, H₃O⁺ is the conjugate acid, and Cl⁻ is the conjugate base.
In another example:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
NH₃ acts as the base, H₂O as the acid, NH₄⁺ is the conjugate acid, and OH⁻ is the conjugate base.
Quick Tip: To identify conjugate pairs, look for species that differ by just one H⁺. For example, NH₃/NH₄⁺ and H₂O/OH⁻ are conjugate pairs.

Access to all documents
Improve your grades
Join milions of students
The Lewis definition provides the most general way to understand acids and bases. A Lewis base is a substance that can donate a pair of electrons, while a Lewis acid accepts a pair of electrons.
This definition broadens our understanding beyond just proton transfer. A Lewis acid-base reaction involves the formation of a coordinate covalent bond, where both electrons in the new bond come from the same atom.
For example, when boron trifluoride (BF₃) reacts with ammonia (NH₃):
F₃B + :NH₃ → F₃B-NH₃
The nitrogen atom in ammonia donates its lone pair of electrons to form a bond with boron.
This definition explains reactions that don't involve hydrogen ions at all, making it the most comprehensive acid-base theory. It can describe interactions between metal ions and ligands, which is crucial in understanding coordination chemistry.
Why This Matters: The Lewis definition explains reactions in organic chemistry, biochemistry, and materials science that wouldn't be considered acid-base reactions under earlier models!

Access to all documents
Improve your grades
Join milions of students
Water is special because it can act as both an acid and a base - a property called amphoteric. It can donate protons (acting as an acid) or accept them (acting as a base) depending on what it's reacting with.
Water undergoes a process called autoionization:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equilibrium has a constant called Kw:
Kw = [H₃O⁺][OH⁻] = 1 × 10⁻¹⁴ at 25°C
In pure water, [H₃O⁺] = [OH⁻] = 1 × 10⁻⁷ M, which creates a perfect balance. This balance determines whether a solution is:
The relationship between these ions is always governed by Kw, meaning if one concentration changes, the other must change to maintain their product at 1 × 10⁻¹⁴.
Think About It: Even in highly acidic solutions, there are still some OH⁻ ions present, just at very low concentrations!

Access to all documents
Improve your grades
Join milions of students
The pH scale provides a convenient way to express the acidity or basicity of a solution without writing out scientific notation. The pH is defined as:
pH = -log[H⁺] or pH = -log[H₃O⁺]
This logarithmic scale means that each unit change in pH represents a tenfold change in hydrogen ion concentration. For instance, a solution with pH 3 has ten times more hydrogen ions than a solution with pH 4.
On this scale at 25°C:
Common substances have characteristic pH values: stomach acid (~1), orange juice (~3.5), pure water (7), blood (~7.4), and household ammonia (~11.5).
We can also express hydroxide concentration using pOH:
pOH = -log[OH⁻]
At 25°C, pH and pOH are related by:
pH + pOH = 14
Real-World Application: Your body maintains blood pH between 7.35-7.45 - even small deviations can be life-threatening, showing how critical pH balance is to biological systems!

Access to all documents
Improve your grades
Join milions of students
Strong acids and bases completely dissociate in water, meaning essentially 100% of their molecules break apart into ions. This dissociation is so complete that it's not considered an equilibrium process.
Common strong acids include:
Common strong bases include:
For strong acids, the hydronium ion concentration [H₃O⁺] equals the initial concentration of the acid. Similarly, for strong bases, the hydroxide concentration [OH⁻] equals the initial concentration multiplied by the number of hydroxide ions per formula unit.
For example, in a 0.057 M HBr solution:
HBr(aq) + H₂O(l) → H₃O⁺(aq) + Br⁻(aq)
[H₃O⁺] = 0.057 M, giving a pH of 1.24
Important: Remember that only a few acids and bases are strong - most are weak and follow equilibrium principles!

Access to all documents
Improve your grades
Join milions of students
Weak acids only partially ionize in water, establishing an equilibrium between the acid and its ions. The extent of this ionization depends on both the acid's concentration and its acid ionization constant, Ka.
For a weak acid HA:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
The acid ionization constant is:
Ka = [H₃O⁺][A⁻]/[HA]
Common weak acids include acetic acid , formic acid , and hydrofluoric acid .
To calculate the pH of a weak acid solution, we set up an equilibrium table with:
If Ka is small compared to the initial concentration, we can use the approximation:
Ka ≈ x²/Ci
This approximation is valid if x < 5% of the initial acid concentration.
Problem-Solving Tip: When calculating weak acid pH, start by assuming x is small. If your answer shows x > 5% of the initial concentration, you'll need to solve the quadratic equation without the approximation!

Access to all documents
Improve your grades
Join milions of students
Similar to weak acids, weak bases only partially ionize in water. Their ionization is characterized by the base ionization constant, Kb.
For a generic weak base B:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The base ionization constant is:
Kb = [BH⁺][OH⁻]/[B]
Common weak bases include ammonia , methylamine , and pyridine .
The approach for solving weak base problems mirrors that for weak acids, except that we solve for [OH⁻] rather than [H₃O⁺]. Once we know [OH⁻], we can calculate pOH and then pH.
For example, if a 0.50 M weak base solution has pH 9.59, we can determine that:
Real-Life Connection: Many medications and household products are weak bases. Understanding their chemistry helps pharmacists formulate drugs and chemists develop effective cleaning products!

Access to all documents
Improve your grades
Join milions of students
There's an inverse relationship between the strength of an acid or base and its conjugate. A strong acid will have a weak conjugate base since it readily gives up its proton. Conversely, a weak acid will have a strong conjugate base that's eager to reclaim a proton.
For example:
This relationship can be quantified. For any conjugate acid-base pair, the product of their ionization constants equals the water ionization constant:
Ka × Kb = Kw = 1 × 10⁻¹⁴
This means if we know the Ka of an acid, we can calculate the Kb of its conjugate base:
Kb = Kw/Ka
For example, if benzoic acid has Ka = 6.5 × 10⁻⁵, then its conjugate base (benzoate ion) has Kb = 1.5 × 10⁻¹⁰.
Study Strategy: When you learn the strength of an acid, you automatically know something about its conjugate base. This pattern helps you predict chemical behavior without memorizing every value!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
6
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
Learn about the reactivity and properties of metals, alkali metals, alkaline earth metals, and transition metals in the periodic table.
Explore the concept of isotopes in chemistry, focusing on atomic number, mass number, and the differences in neutron count. This summary provides a clear definition and examples to enhance your understanding of isotopes and their significance in the study of elements.
This note contains the summary of the topics redox reaction, electrochemistry, nuclear chemistry, and stoichiometry.
ionic and covalent compounds
Learn about Henry Moseley's contribution to the modern periodic table and the arrangement of elements based on atomic number and properties.
An introduction to chemistry including the different branches of chemistry.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user