Chemistry is the study of matter and energy, exploring how... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Knowunity AI
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
73
•
Feb 12, 2026
•
Isabella
@isabellad
Chemistry is the study of matter and energy, exploring how... Show more

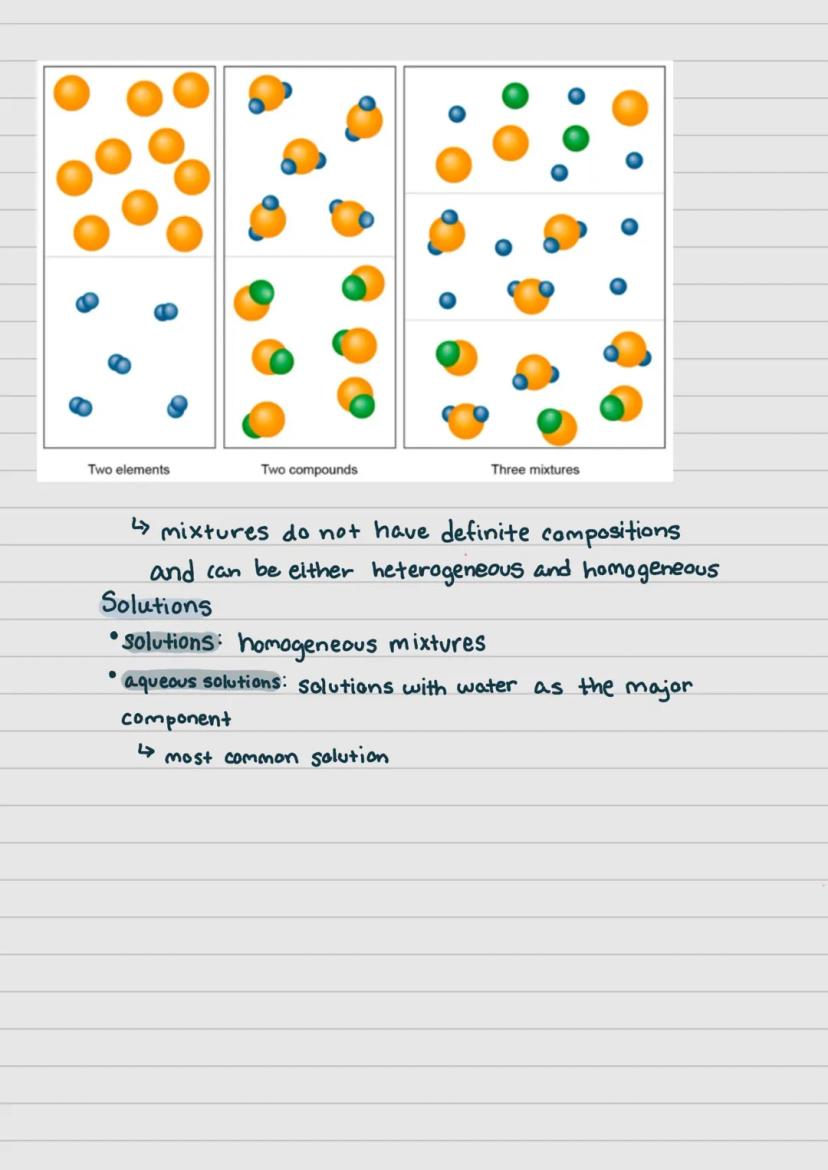





The world around you is made of matter - anything that has mass and occupies space. In chemistry, we study how this matter behaves and changes. All materials on Earth are composed of about 100 different elements, which are the simplest forms of matter with distinct properties that cannot be broken down further.
When you look at pure substances, you're dealing with either elements or compounds. An atom is the smallest amount of an element that still maintains that element's characteristics. When atoms form chemical bonds with other atoms, they create compounds - substances with their own unique properties and definite compositions.
The key difference between elements and compounds is that compounds always contain the same elements in the same proportions, regardless of their source. This consistency is what makes chemistry predictable and allows scientists to identify substances reliably.
Remember this! Pure substances (elements and compounds) cannot be separated by physical means, while mixtures can. This distinction is crucial for understanding chemical reactions.
When two or more pure substances are physically combined without chemical bonding, they form a mixture. Unlike compounds, mixtures don't have definite compositions and can be separated using physical methods.
Mixtures come in two main varieties: heterogeneous and homogeneous. In heterogeneous mixtures, you can visually distinguish the different components (like oil and water). In homogeneous mixtures, the components are evenly distributed and appear uniform.
Solutions are homogeneous mixtures where one substance is dissolved in another. The most common type is an aqueous solution, where water serves as the major component. Think of dissolving sugar in water - you can't see the sugar particles, but they're distributed throughout.

Every substance has a unique set of properties that helps us identify it. These characteristics fall into two main categories: physical and chemical properties.
Physical properties can be observed or measured without changing the substance's identity. These include length, mass, volume, color, and density. When you describe how water looks clear or how metal feels cold, you're noting physical properties.
Chemical properties only become evident during chemical reactions. Flammability (ability to burn) is a classic example - you only know if something is flammable when you try to ignite it. Chemical properties tell us how a substance might change into something new.
Quick tip: Remember the difference between extensive and intensive properties! Extensive properties (like mass) depend on sample size, while intensive properties (like density) remain constant regardless of how much of the substance you have.

When matter changes, it undergoes either a physical or chemical transformation. During a physical change, the appearance might change, but the chemical composition stays the same - like ice melting into water.
Chemical changes involve a complete transformation where the original substance (the reactant) becomes a new substance (the product). These changes occur when chemical bonds break or form. Every chemical reaction involves energy changes - when bonds break, energy is absorbed; when bonds form, energy is released.
Understanding these energy dynamics helps explain why some reactions feel cold and others produce heat. For example, when you observe a substance losing mass after heating, it suggests a decomposition reaction where part of the compound has escaped as gas.
When a substance gains mass after reacting with air, it has combined with a gas (likely oxygen) to form a new compound. These observations give chemists clues about what's happening at the molecular level.

Mass measures how much matter exists in an object. It's directly proportional to weight, though weight can vary with gravitational force while mass remains constant regardless of location.
Energy, the capacity to do work, follows a fundamental principle: it cannot be created or destroyed, only converted from one form to another. This concept, known as conservation of energy, parallels the Law of Conservation of Mass, which states that the total mass present before and after any chemical reaction or physical change remains equal.
Science progresses through several levels of understanding. Hypotheses are initial explanations based on observations that must be tested. Theories are broader explanations that connect many observations and have been extensively verified. Scientific laws are statements of phenomena that are always observed to be true.
Think about it: The scientific process isn't linear! Scientists develop hypotheses based on observations, test them rigorously, and sometimes these grow into comprehensive theories that explain why scientific laws exist.

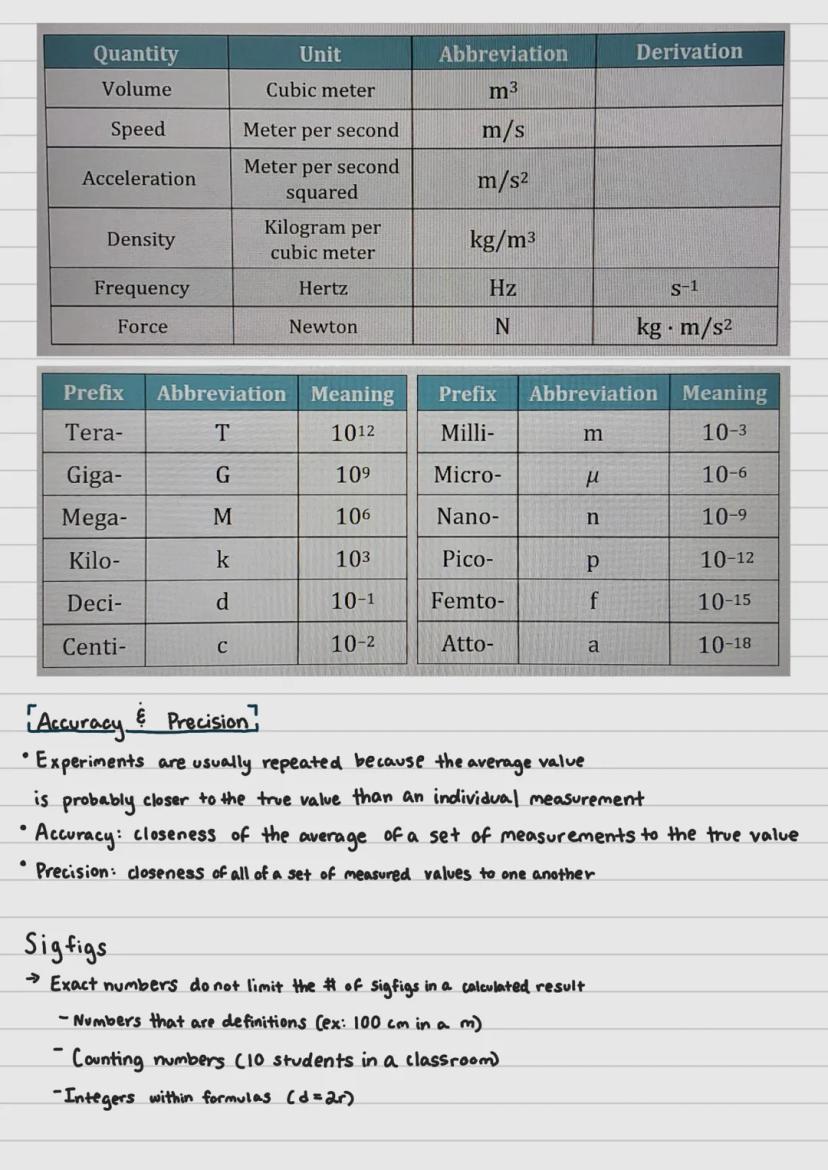
Measurements in chemistry rely on the International System of Units (SI), which provides standard units for seven base quantities. These fundamental measurements include the second (time), meter (length), kilogram (mass), ampere (electric current), kelvin (temperature), mole (amount of substance), and candela (luminous intensity).
Every other measurement in science combines these base units in some way. For instance, volume is measured in cubic meters (m³), and speed is measured in meters per second .
To handle very large or very small measurements, scientists use SI prefixes. These modifiers change the value of the base unit by powers of ten. The prefix "kilo-" multiplies a unit by 1,000, while "centi-" divides it by 100. This system allows scientists to express measurements from the astronomical to the microscopic using the same basic units.
In the scientific world, how you measure matters just as much as what you measure. When scientists repeat experiments, they're looking for consistency in their results.
Accuracy refers to how close your measurements are to the true value, while precision describes how close repeated measurements are to each other. You can think of accuracy as hitting the bullseye on a target, while precision is hitting the same spot repeatedly (even if it's not the bullseye).
When calculating results, scientists use significant figures (sigfigs) to indicate the precision of their measurements. Some numbers, however, don't limit sigfigs in calculations. These include exact numbers like definitions (100 cm in 1 m), counting numbers (10 students), and integers in formulas .
Pro tip: When reporting your lab results, pay attention to both accuracy and precision. A measurement can be precise (consistent) without being accurate (correct), or accurate without being precise!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Isabella
@isabellad
Chemistry is the study of matter and energy, exploring how substances interact and transform. This introduction to chemistry covers the fundamental concepts of matter, measurements, and scientific principles that form the foundation of the subject.

Access to all documents
Improve your grades
Join milions of students
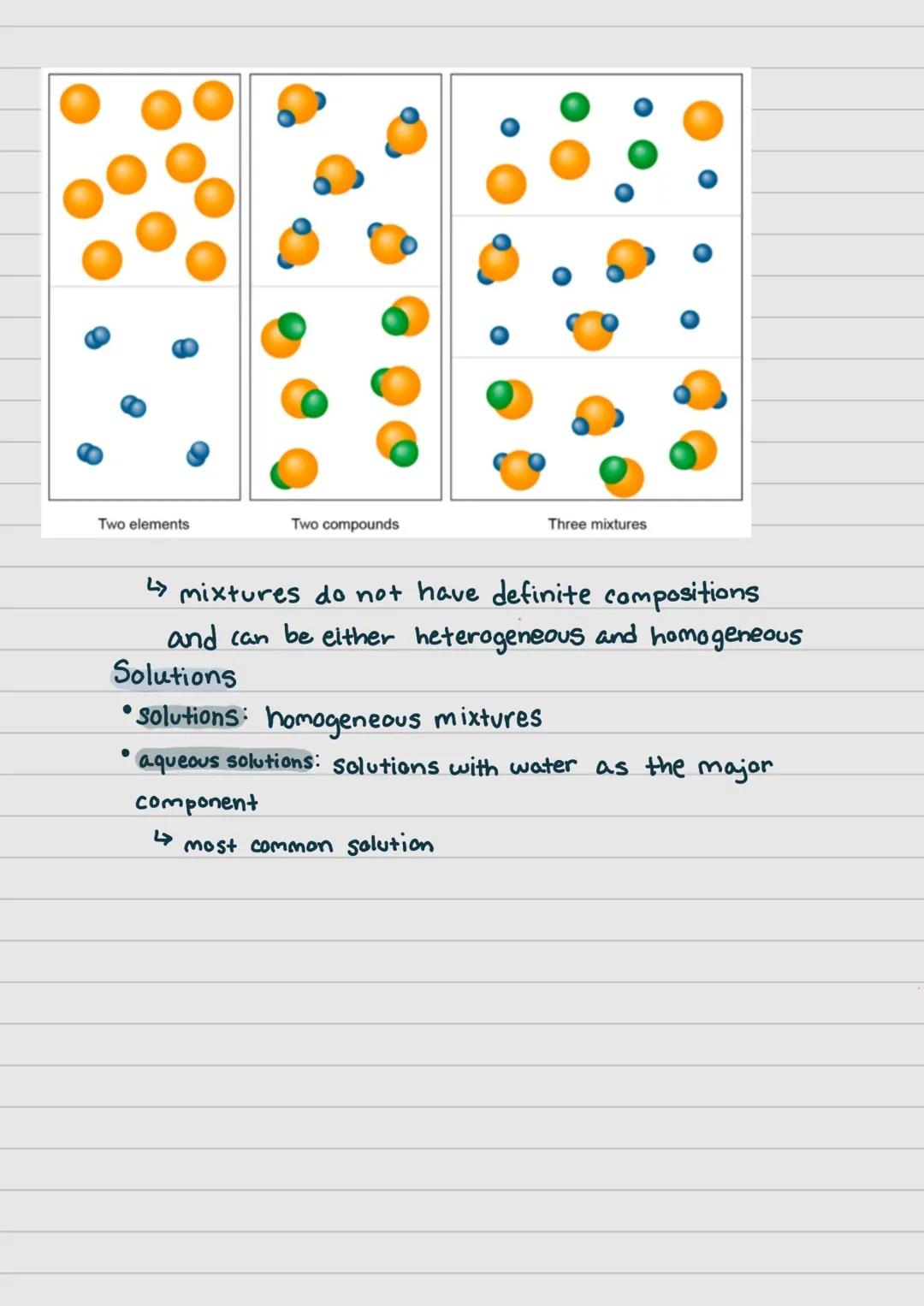
The world around you is made of matter - anything that has mass and occupies space. In chemistry, we study how this matter behaves and changes. All materials on Earth are composed of about 100 different elements, which are the simplest forms of matter with distinct properties that cannot be broken down further.
When you look at pure substances, you're dealing with either elements or compounds. An atom is the smallest amount of an element that still maintains that element's characteristics. When atoms form chemical bonds with other atoms, they create compounds - substances with their own unique properties and definite compositions.
The key difference between elements and compounds is that compounds always contain the same elements in the same proportions, regardless of their source. This consistency is what makes chemistry predictable and allows scientists to identify substances reliably.
Remember this! Pure substances (elements and compounds) cannot be separated by physical means, while mixtures can. This distinction is crucial for understanding chemical reactions.

Access to all documents
Improve your grades
Join milions of students
When two or more pure substances are physically combined without chemical bonding, they form a mixture. Unlike compounds, mixtures don't have definite compositions and can be separated using physical methods.
Mixtures come in two main varieties: heterogeneous and homogeneous. In heterogeneous mixtures, you can visually distinguish the different components (like oil and water). In homogeneous mixtures, the components are evenly distributed and appear uniform.
Solutions are homogeneous mixtures where one substance is dissolved in another. The most common type is an aqueous solution, where water serves as the major component. Think of dissolving sugar in water - you can't see the sugar particles, but they're distributed throughout.

Access to all documents
Improve your grades
Join milions of students
Every substance has a unique set of properties that helps us identify it. These characteristics fall into two main categories: physical and chemical properties.
Physical properties can be observed or measured without changing the substance's identity. These include length, mass, volume, color, and density. When you describe how water looks clear or how metal feels cold, you're noting physical properties.
Chemical properties only become evident during chemical reactions. Flammability (ability to burn) is a classic example - you only know if something is flammable when you try to ignite it. Chemical properties tell us how a substance might change into something new.
Quick tip: Remember the difference between extensive and intensive properties! Extensive properties (like mass) depend on sample size, while intensive properties (like density) remain constant regardless of how much of the substance you have.

Access to all documents
Improve your grades
Join milions of students
When matter changes, it undergoes either a physical or chemical transformation. During a physical change, the appearance might change, but the chemical composition stays the same - like ice melting into water.
Chemical changes involve a complete transformation where the original substance (the reactant) becomes a new substance (the product). These changes occur when chemical bonds break or form. Every chemical reaction involves energy changes - when bonds break, energy is absorbed; when bonds form, energy is released.
Understanding these energy dynamics helps explain why some reactions feel cold and others produce heat. For example, when you observe a substance losing mass after heating, it suggests a decomposition reaction where part of the compound has escaped as gas.
When a substance gains mass after reacting with air, it has combined with a gas (likely oxygen) to form a new compound. These observations give chemists clues about what's happening at the molecular level.

Access to all documents
Improve your grades
Join milions of students
Mass measures how much matter exists in an object. It's directly proportional to weight, though weight can vary with gravitational force while mass remains constant regardless of location.
Energy, the capacity to do work, follows a fundamental principle: it cannot be created or destroyed, only converted from one form to another. This concept, known as conservation of energy, parallels the Law of Conservation of Mass, which states that the total mass present before and after any chemical reaction or physical change remains equal.
Science progresses through several levels of understanding. Hypotheses are initial explanations based on observations that must be tested. Theories are broader explanations that connect many observations and have been extensively verified. Scientific laws are statements of phenomena that are always observed to be true.
Think about it: The scientific process isn't linear! Scientists develop hypotheses based on observations, test them rigorously, and sometimes these grow into comprehensive theories that explain why scientific laws exist.

Access to all documents
Improve your grades
Join milions of students
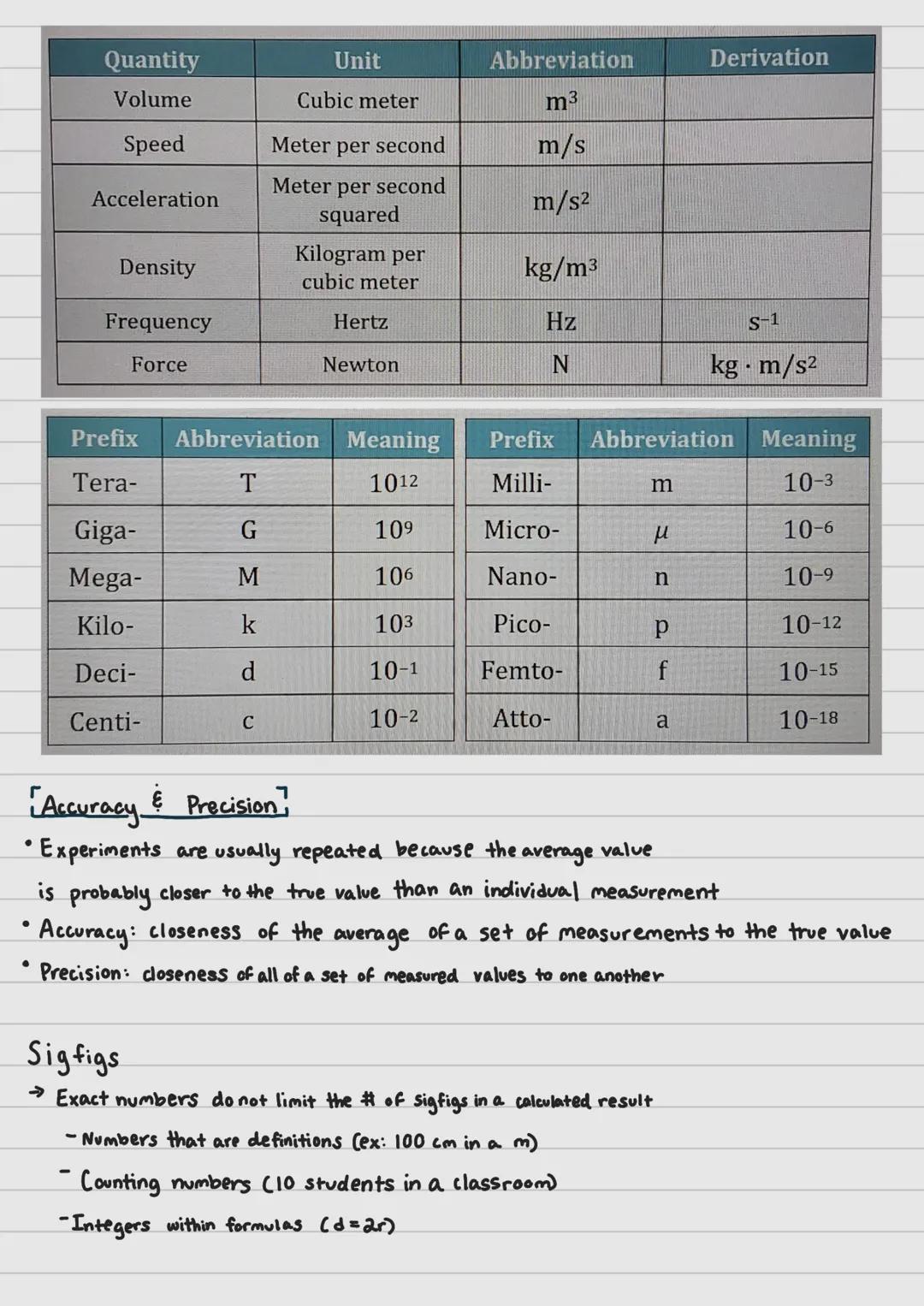
Measurements in chemistry rely on the International System of Units (SI), which provides standard units for seven base quantities. These fundamental measurements include the second (time), meter (length), kilogram (mass), ampere (electric current), kelvin (temperature), mole (amount of substance), and candela (luminous intensity).
Every other measurement in science combines these base units in some way. For instance, volume is measured in cubic meters (m³), and speed is measured in meters per second .
To handle very large or very small measurements, scientists use SI prefixes. These modifiers change the value of the base unit by powers of ten. The prefix "kilo-" multiplies a unit by 1,000, while "centi-" divides it by 100. This system allows scientists to express measurements from the astronomical to the microscopic using the same basic units.

Access to all documents
Improve your grades
Join milions of students
In the scientific world, how you measure matters just as much as what you measure. When scientists repeat experiments, they're looking for consistency in their results.
Accuracy refers to how close your measurements are to the true value, while precision describes how close repeated measurements are to each other. You can think of accuracy as hitting the bullseye on a target, while precision is hitting the same spot repeatedly (even if it's not the bullseye).
When calculating results, scientists use significant figures (sigfigs) to indicate the precision of their measurements. Some numbers, however, don't limit sigfigs in calculations. These include exact numbers like definitions (100 cm in 1 m), counting numbers (10 students), and integers in formulas .
Pro tip: When reporting your lab results, pay attention to both accuracy and precision. A measurement can be precise (consistent) without being accurate (correct), or accurate without being precise!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
1
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
AP Chem
notes about elements, compounds, mixtures, and their properties
Explains differences between pure substances & mixtures. Explains differences between solutions & heterogeneous mixture. Describes rules that are used to count the number of significant figures.
Term 1 Unit 1-5
Notes relating to CED of AP Chemistry of 1.3 - 1.6
How different scientists contributed to atomic theory and our modern understanding of what an atom is.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user