Molecular geometry describes the 3D arrangement of atoms in molecules,... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
111
•
Feb 2, 2026
•
Saryna Cadet
@sarynacad_g5eut
Molecular geometry describes the 3D arrangement of atoms in molecules,... Show more
Ever wonder why some molecules can dissolve in water while others can't? It all comes down to their shape! Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule.
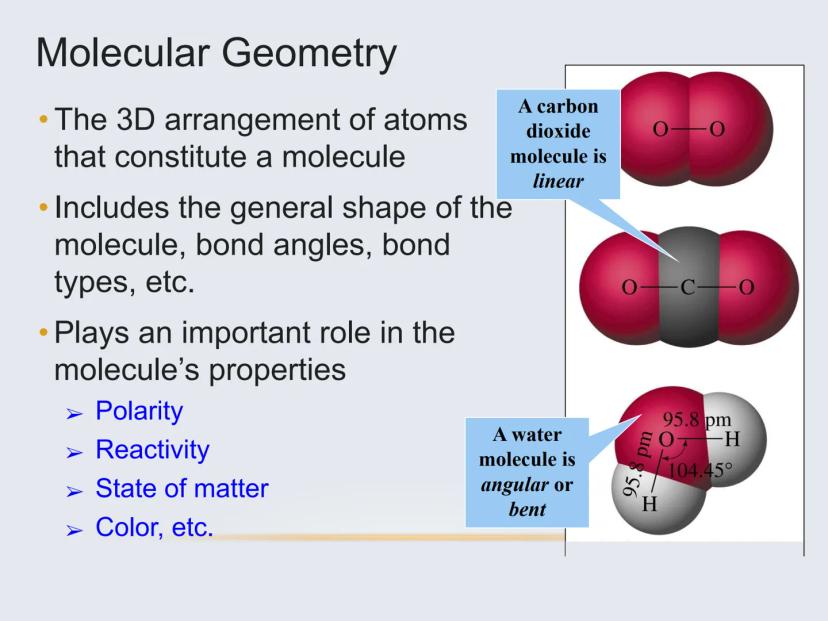
The shape of a molecule isn't just interesting to look at—it directly influences the molecule's properties like polarity, reactivity, and even what state of matter it exists in at room temperature.
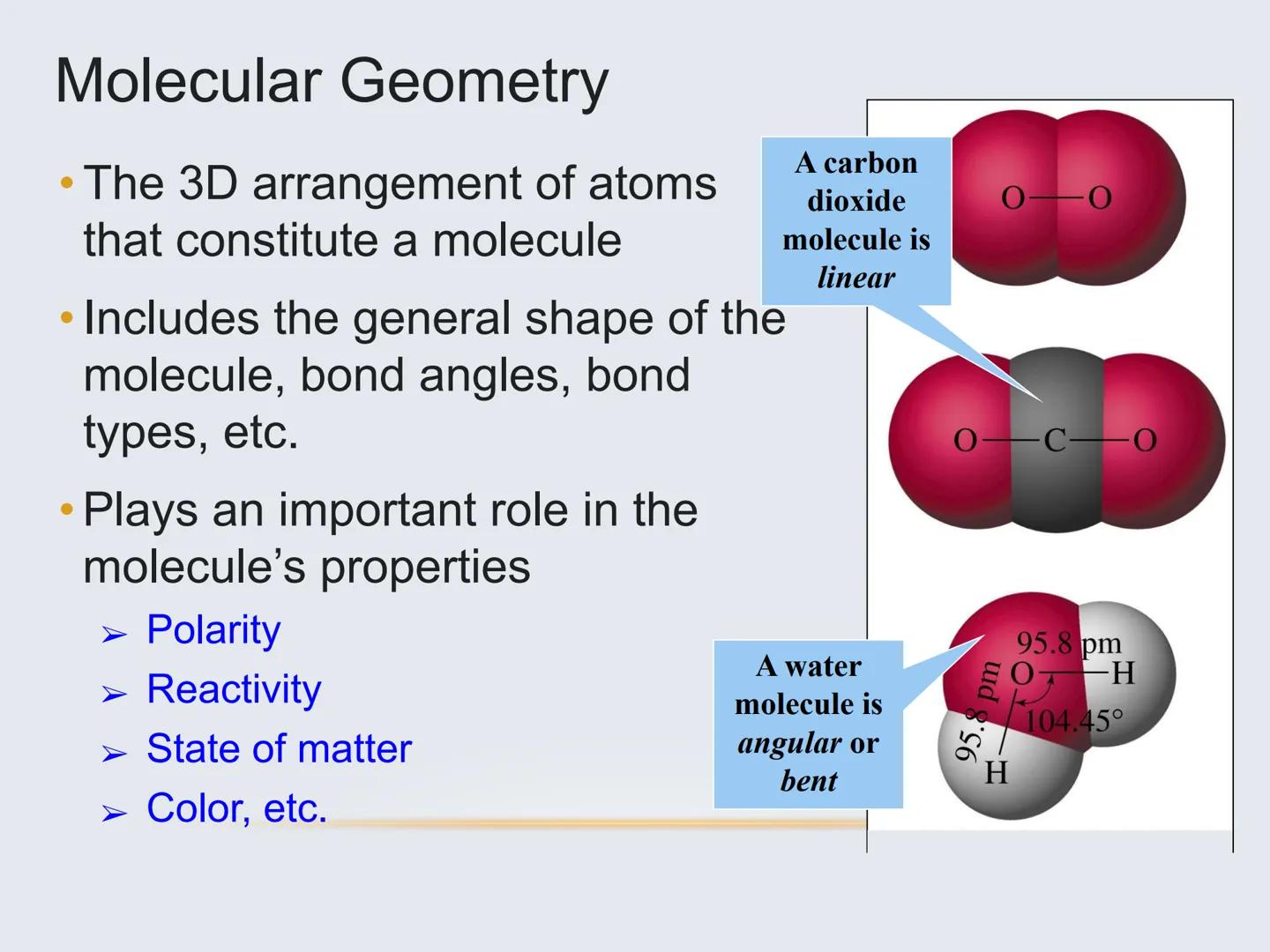
Did you know? Carbon dioxide (CO₂) is linear, while water (H₂O) is bent. These different shapes explain why water is a liquid at room temperature while CO₂ is a gas!
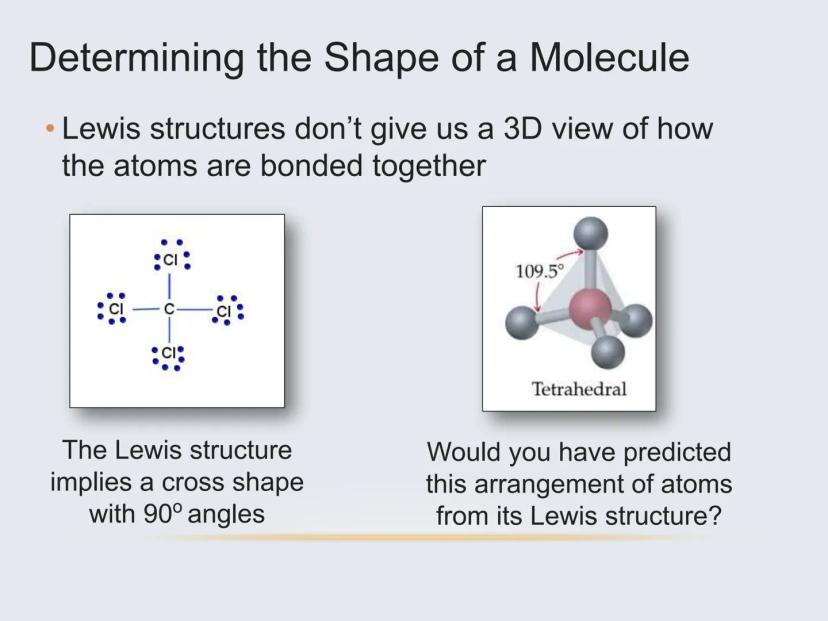
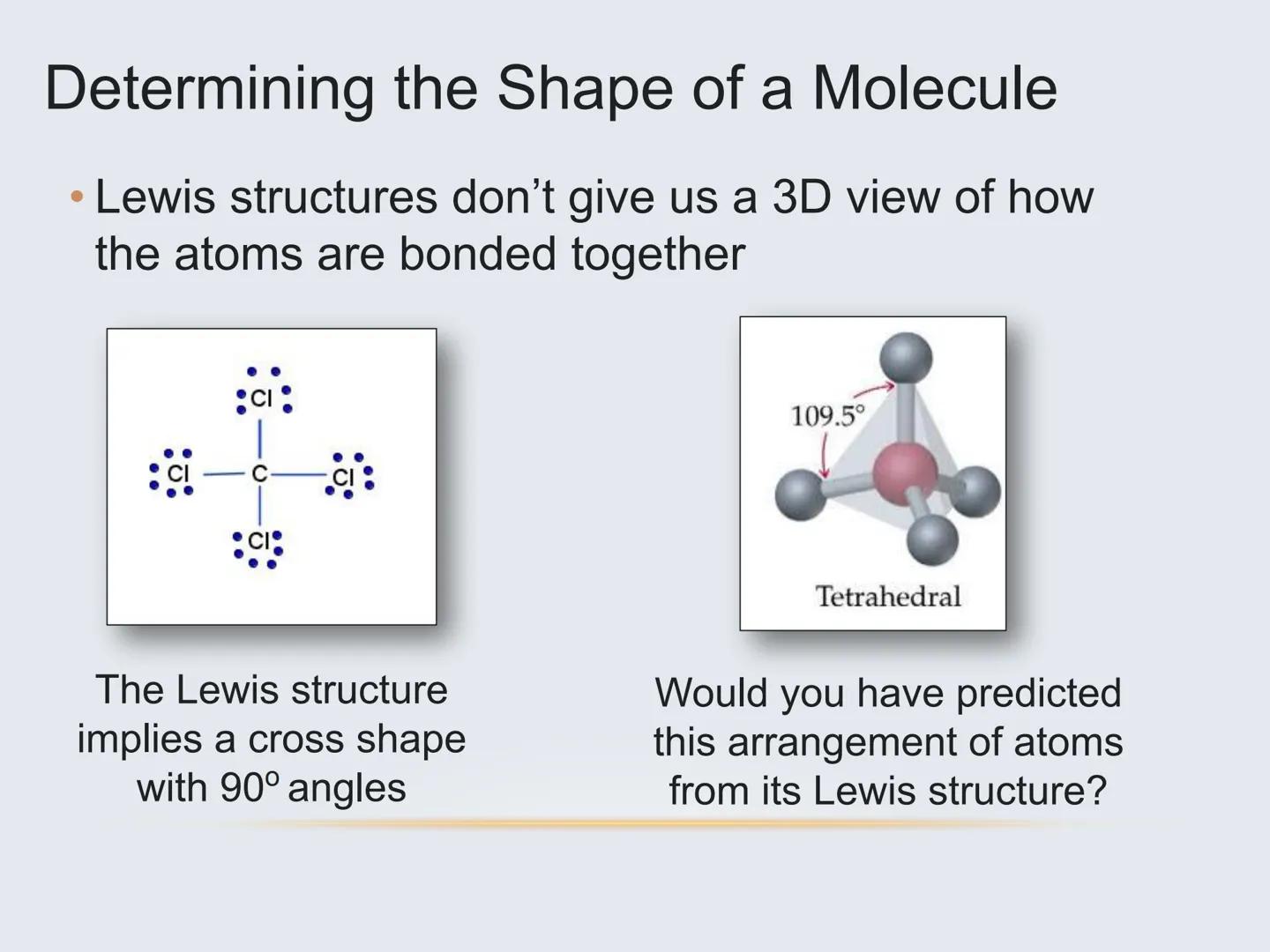
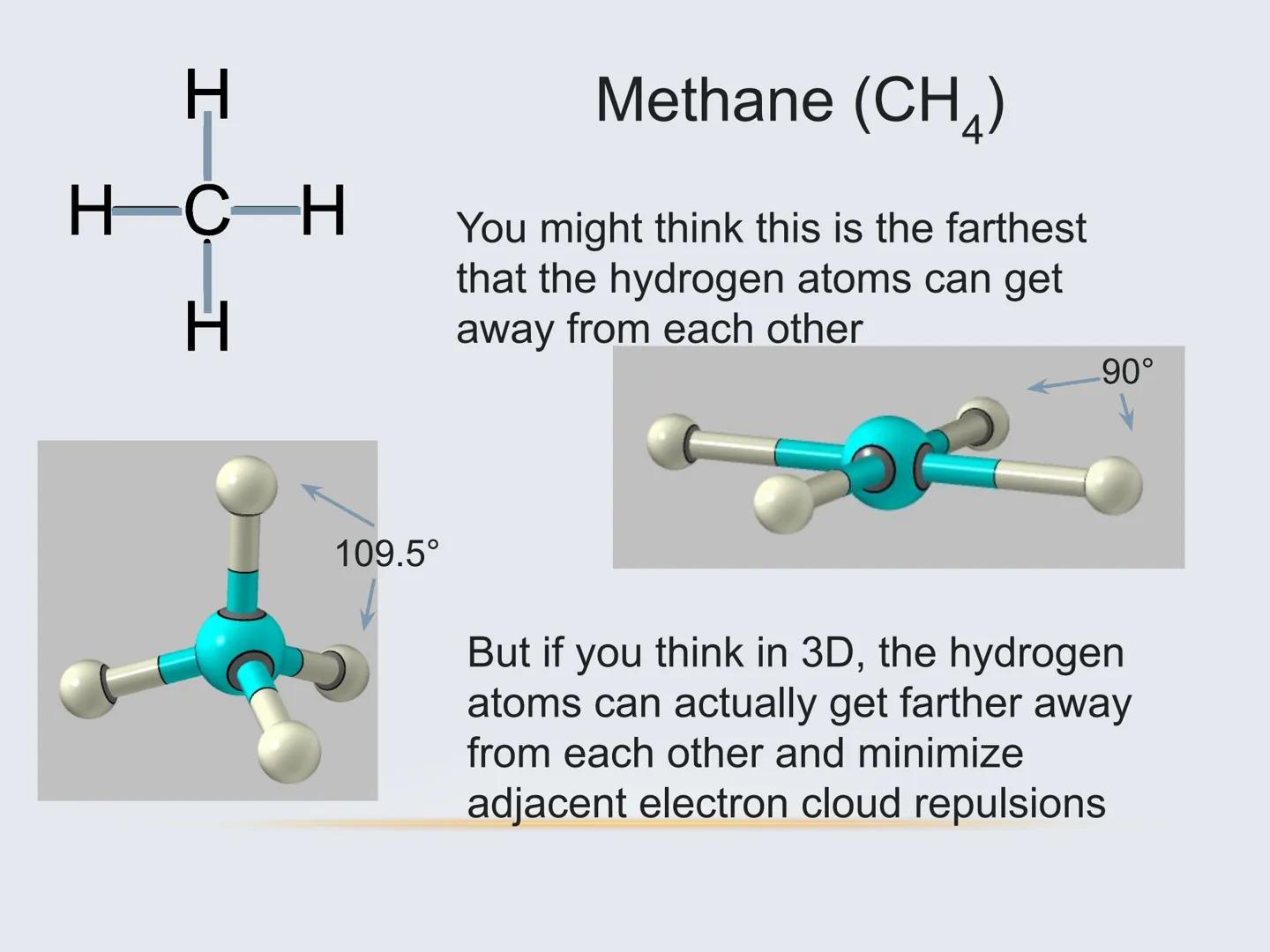
The Lewis structure of a molecule (those dot diagrams you've drawn) doesn't actually tell you how a molecule is arranged in 3D space. The real arrangement is often surprising!
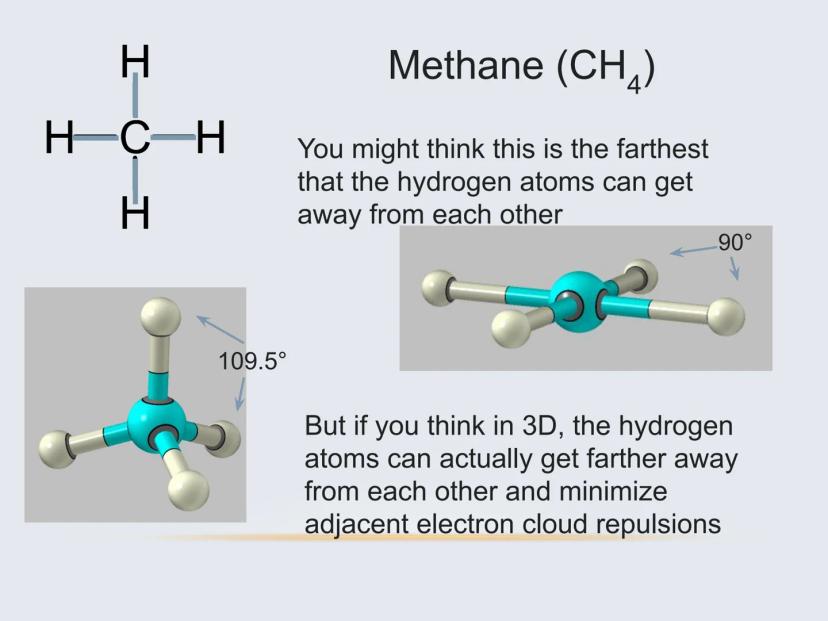
For example, methane (CH₄) might look like a flat cross on paper, but in reality, the hydrogen atoms arrange themselves in a 3D tetrahedral shape with 109.5° angles between bonds. This happens because electrons naturally repel each other and seek to be as far apart as possible.
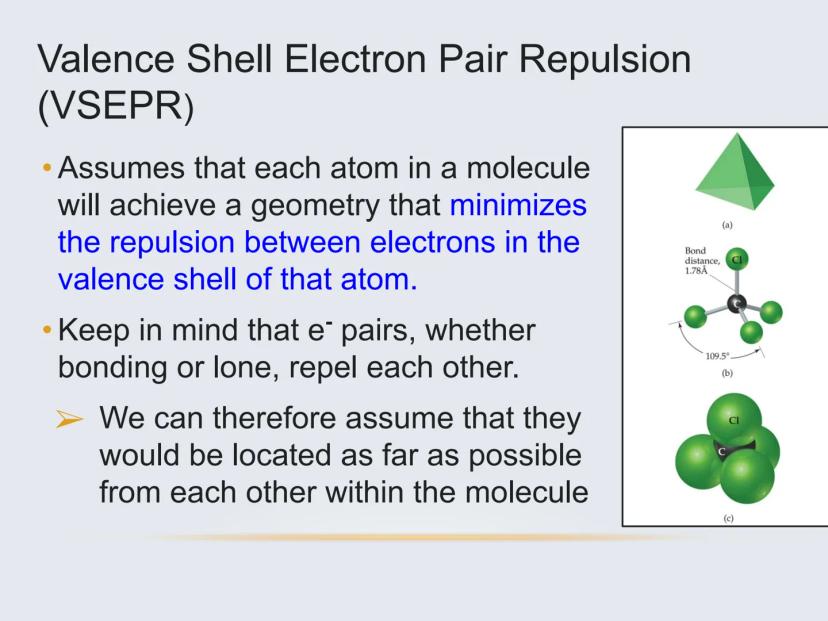
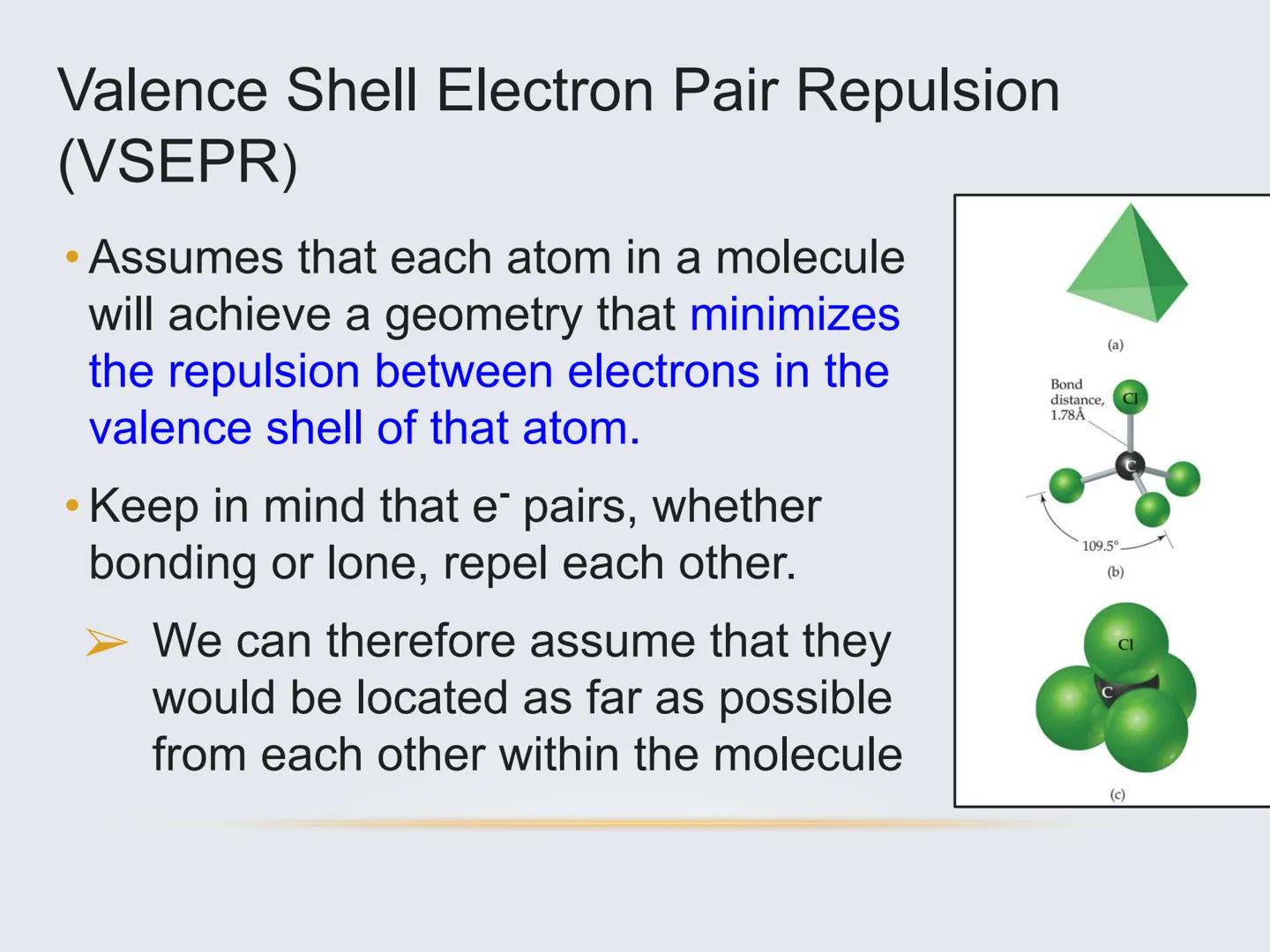
To predict these 3D arrangements, we use a model called VSEPR (Valence Shell Electron Pair Repulsion). The basic idea is simple: electron pairs around an atom, whether they're bonding or non-bonding, push away from each other as much as possible.
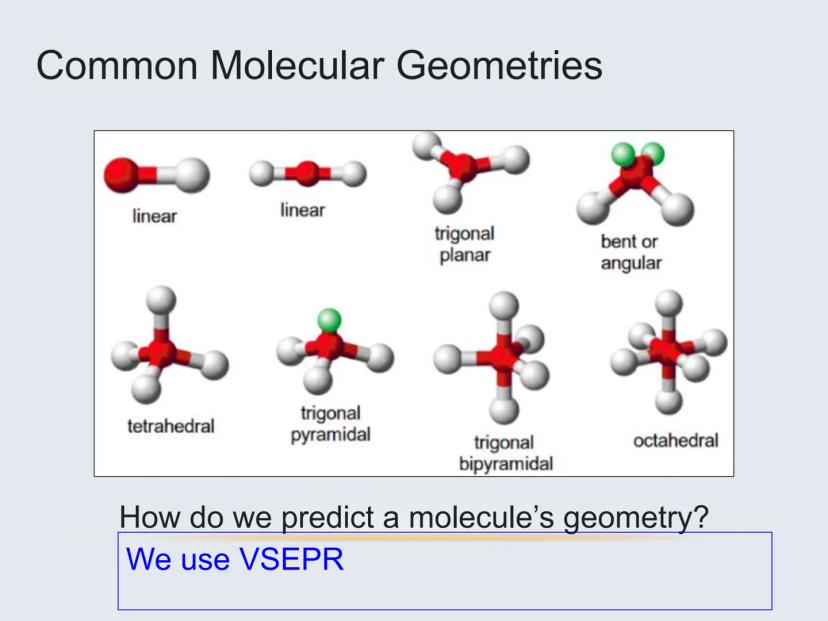
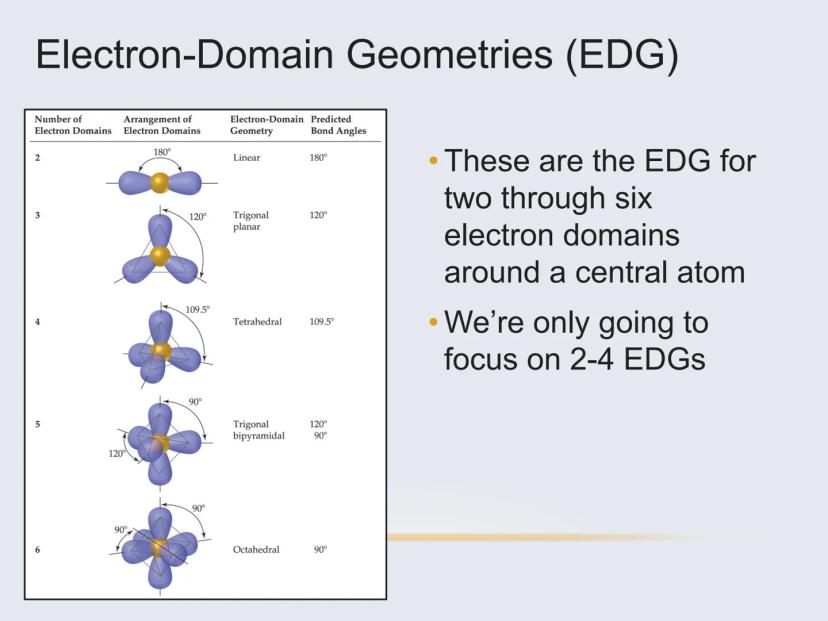
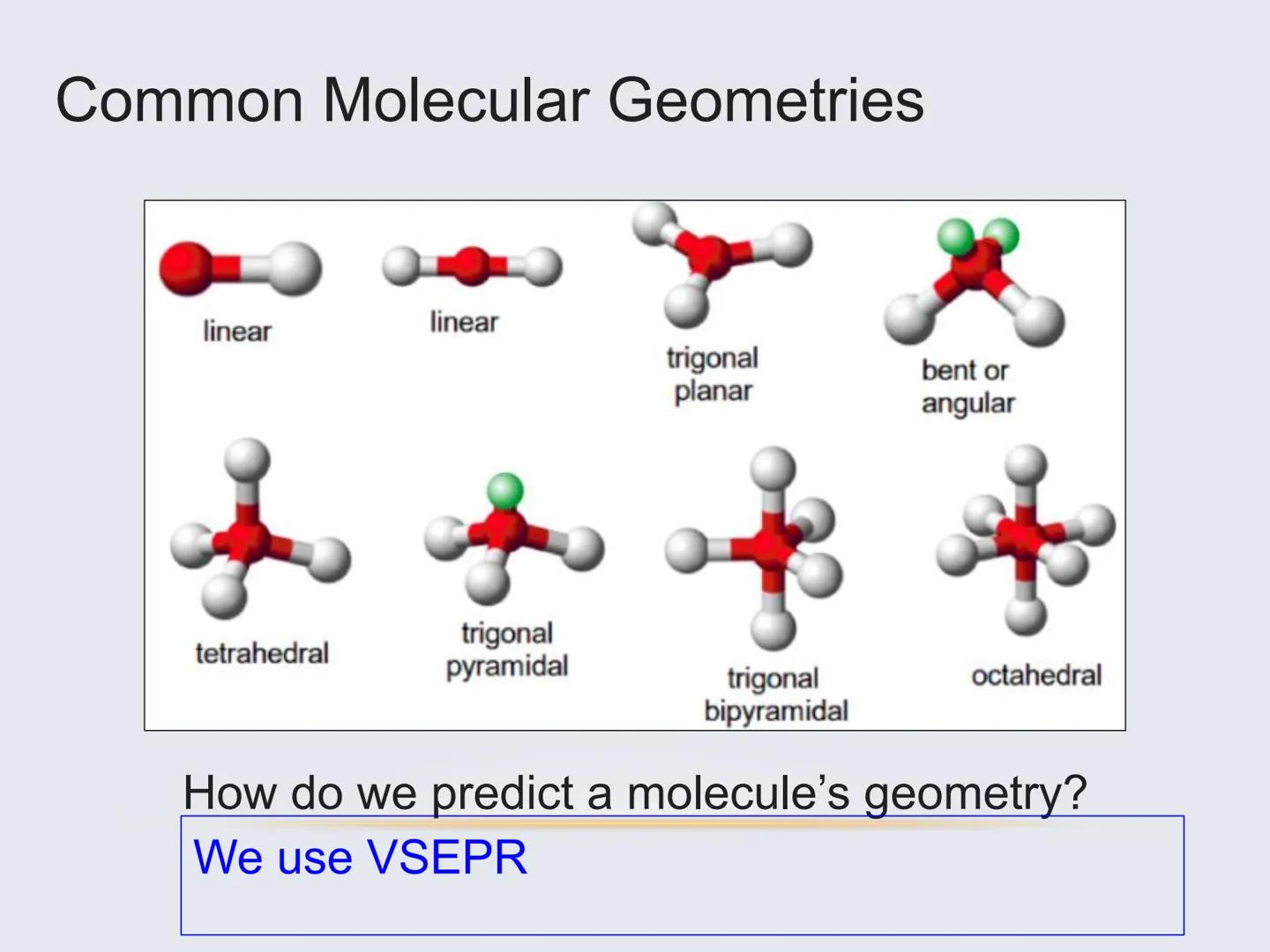
Molecules can take on several common shapes depending on how many atoms and lone pairs surround the central atom:
These shapes aren't random—they're the result of electrons pushing away from each other to minimize repulsion. VSEPR theory helps us predict which shape will form in each case.
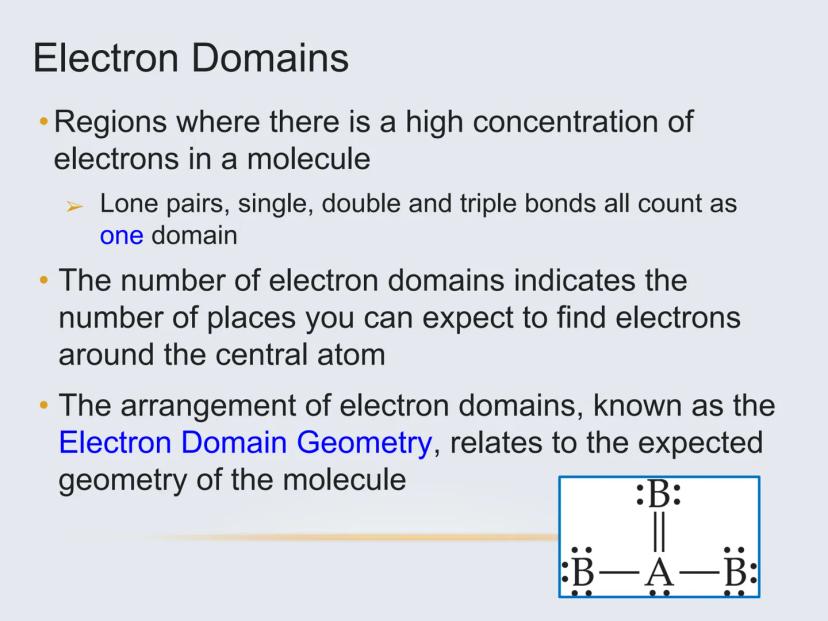
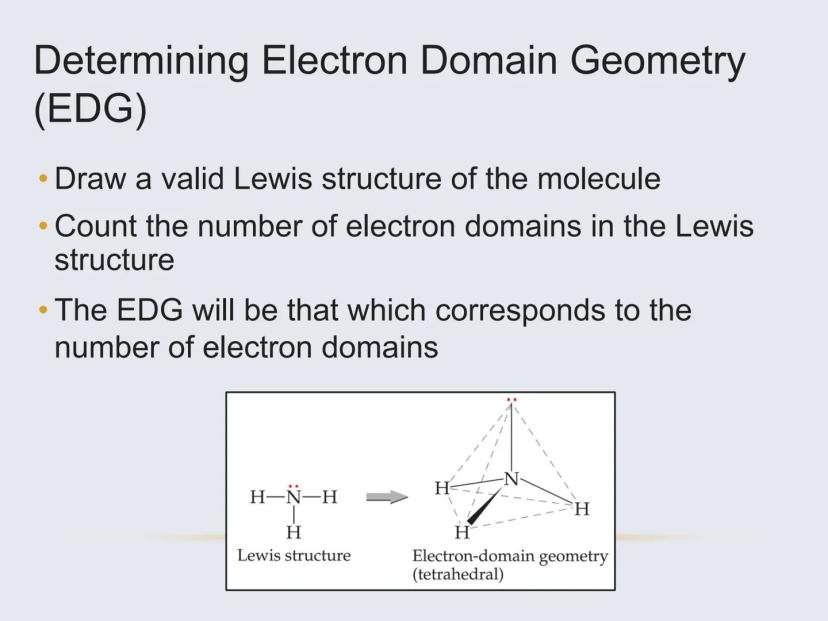
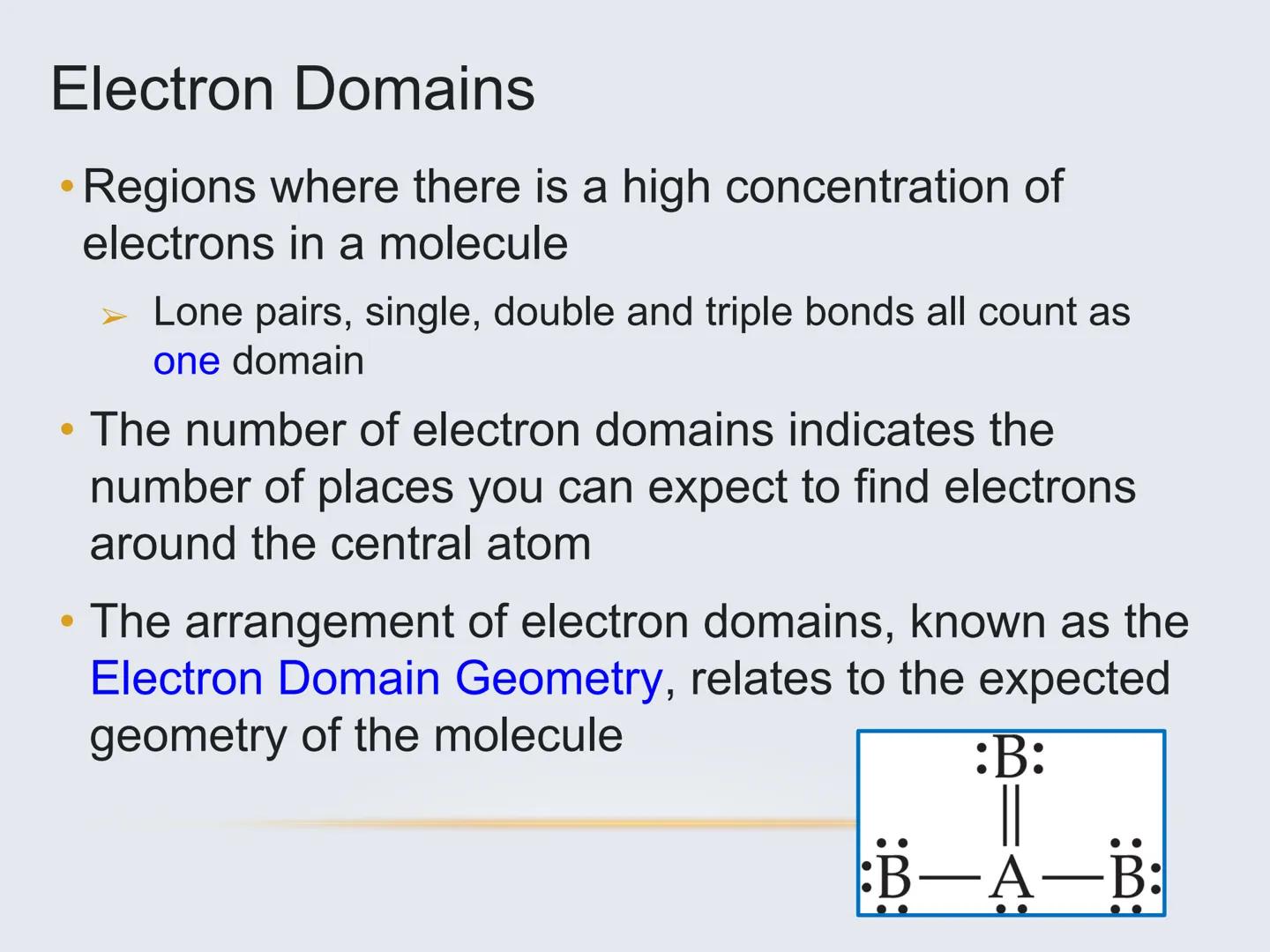
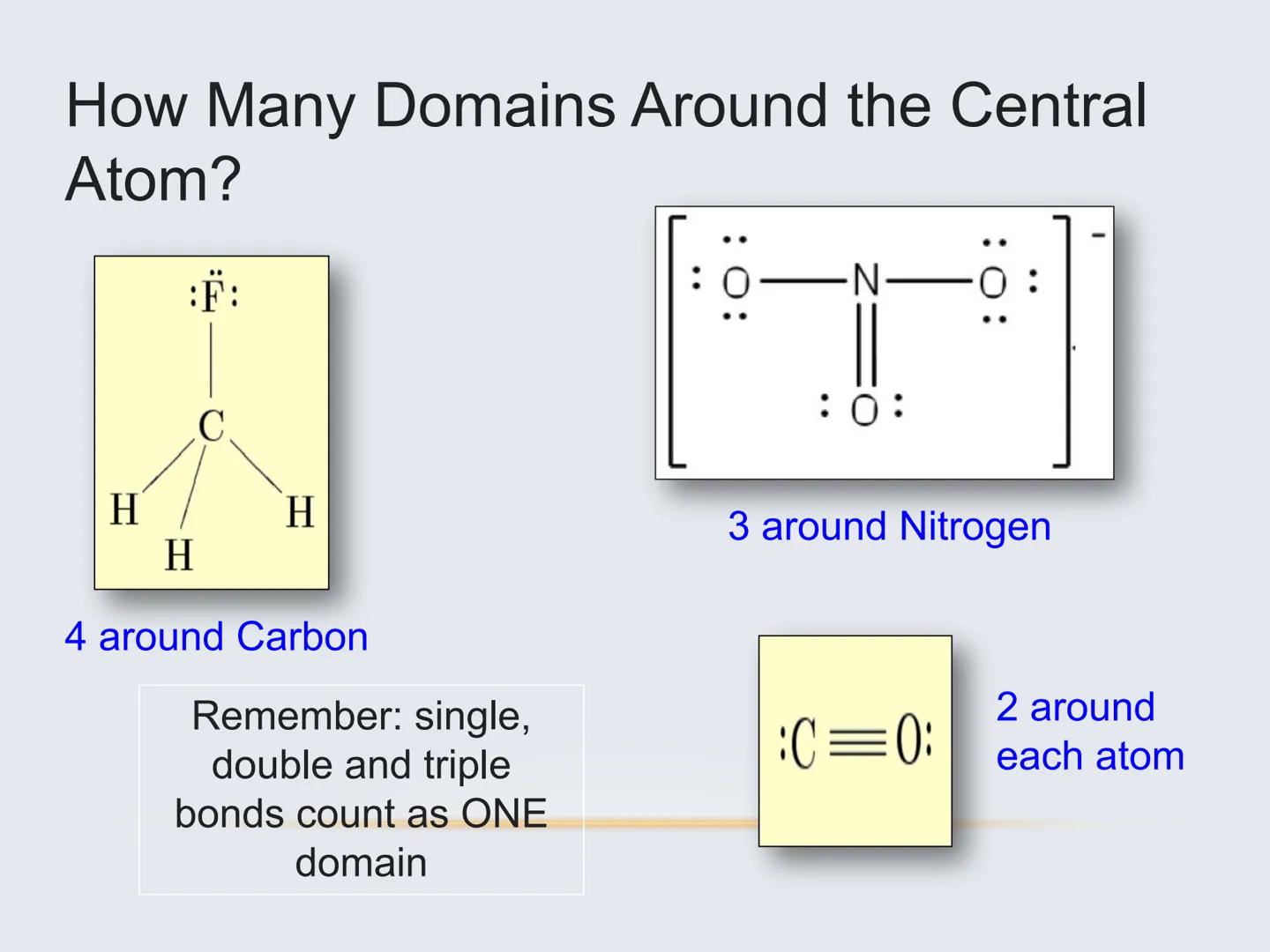
To figure out a molecule's shape, you need to identify its electron domains—regions around the central atom where electrons are concentrated.
An electron domain can be:
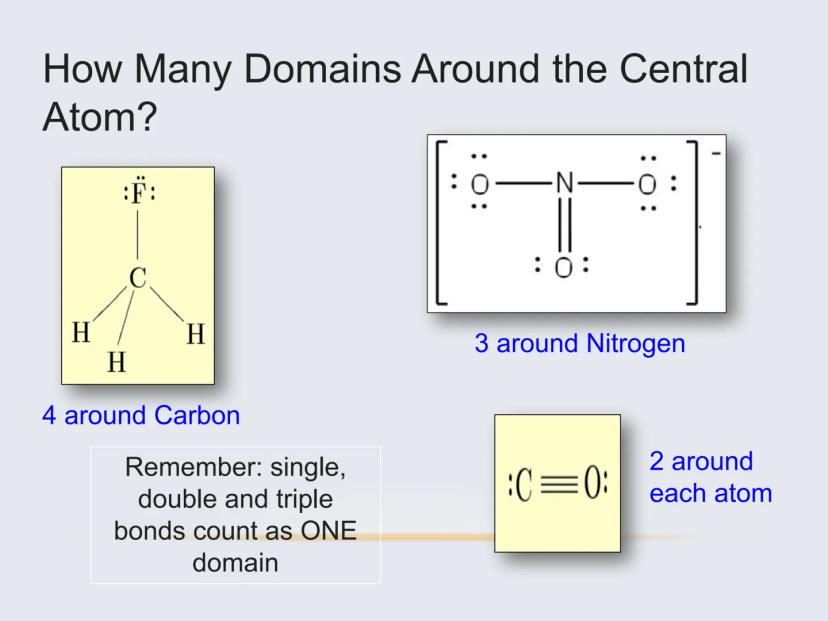
Important: All bonds (single, double, or triple) count as just ONE electron domain. This is key for correctly determining geometry.
For example, carbon dioxide (CO₂) has only two electron domains (the two double bonds), making it linear. Water (H₂O) has four electron domains (two bonds and two lone pairs), making it bent.
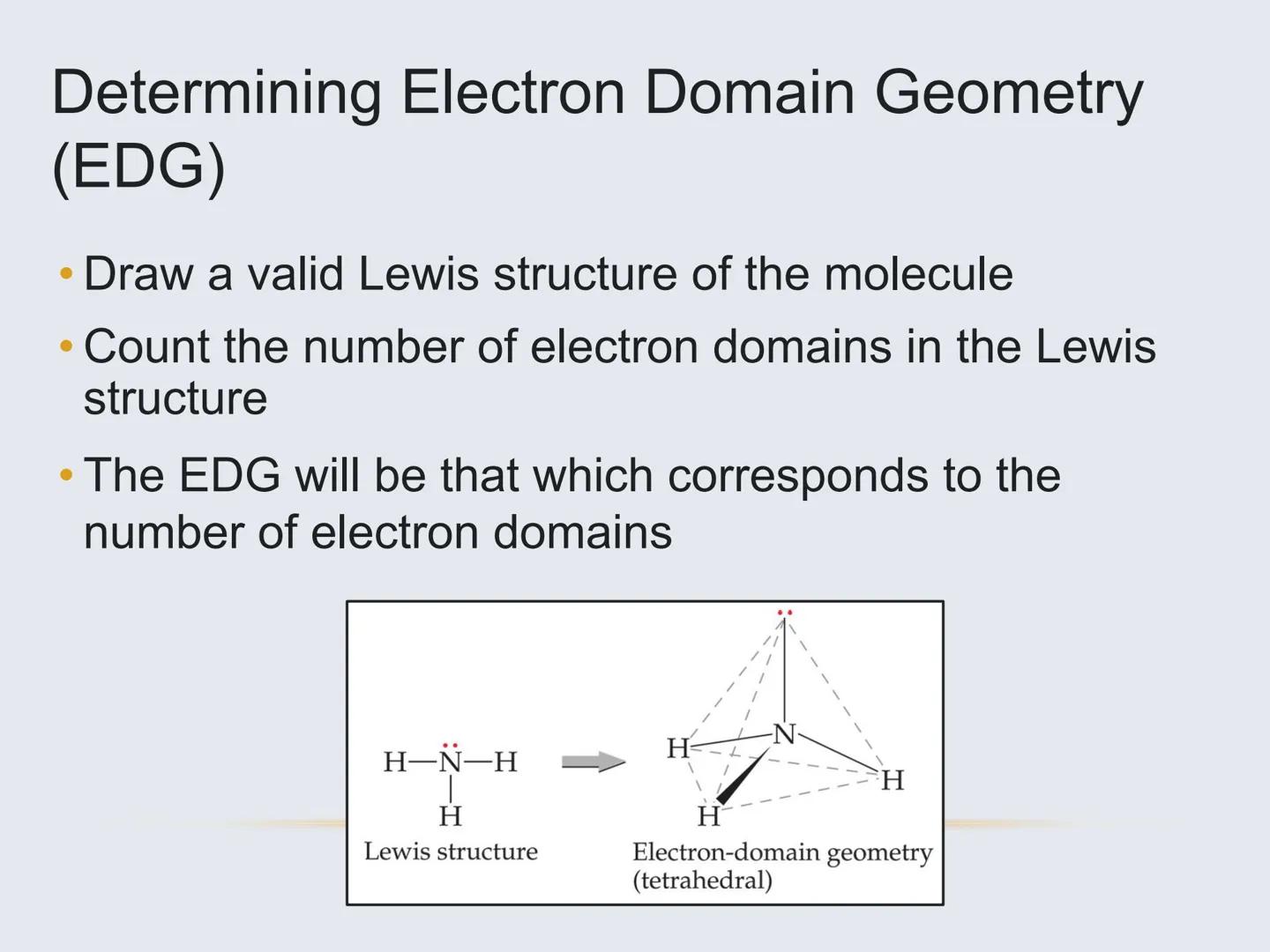
There are two related but different concepts you need to understand:
Electron Domain Geometry (EDG) refers to the arrangement of all electron domains (bonds and lone pairs) around the central atom. It depends only on the total number of electron domains.
Molecular Geometry refers to the actual shape formed by the atoms (not including lone pairs). It depends on both the electron domain geometry and how many of those domains are lone pairs.
For example, ammonia (NH₃) has 4 electron domains (tetrahedral EDG), but since one domain is a lone pair, its molecular geometry is trigonal pyramidal.
Remember: Lone pairs take up space but aren't visible in the final shape!
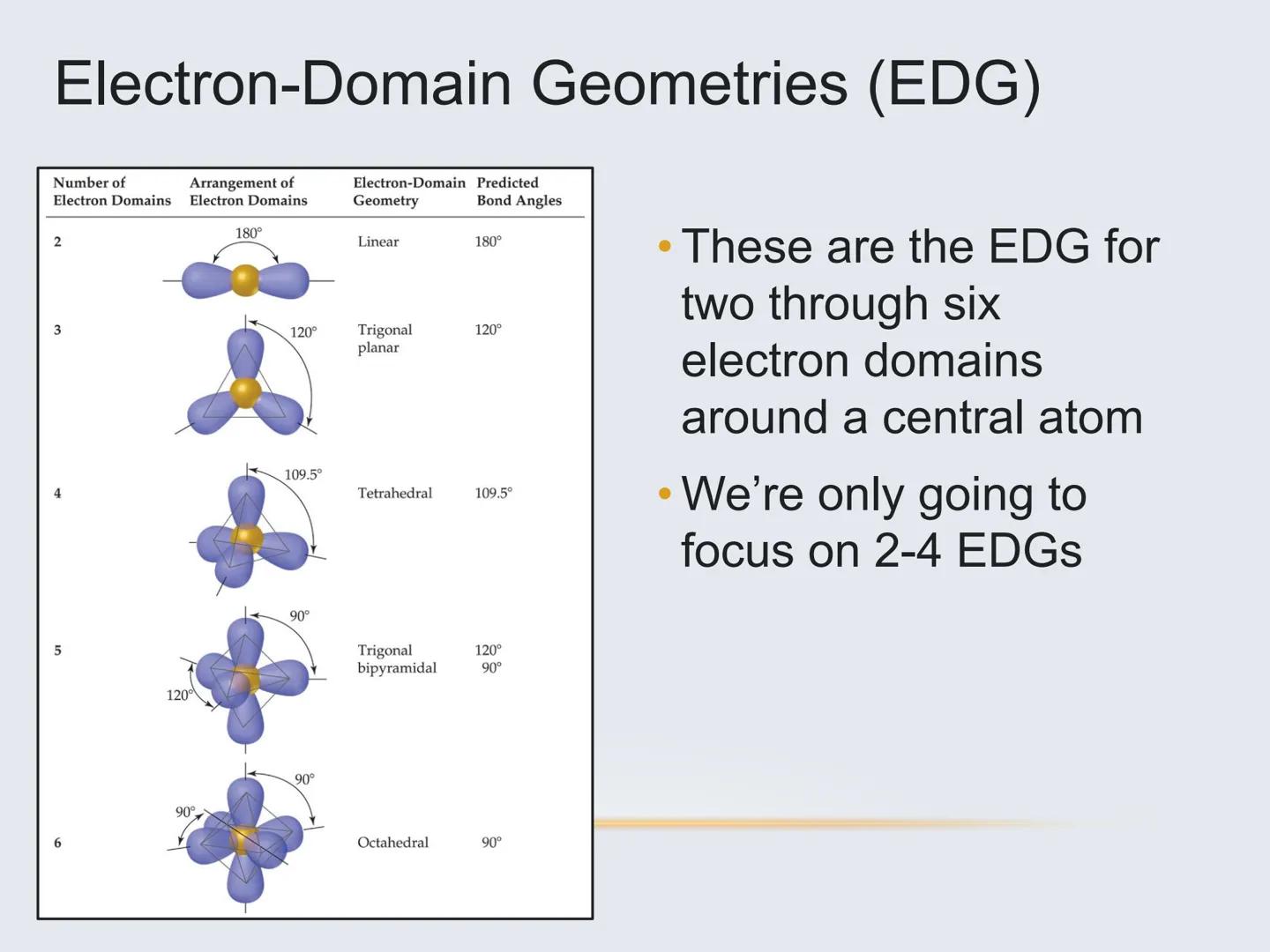
To predict a molecule's shape:
The number of electron domains tells you the basic arrangement:
Bold tip: Remember that the molecular geometry often differs from the electron domain geometry when lone pairs are present!
When a central atom has only two electron domains, the geometry is always linear with a bond angle of 180°.
Examples include:
In these molecules, the two electron domains push as far away from each other as possible, creating a straight line. This arrangement minimizes repulsion between the electron clouds.
Even if these molecules have double or triple bonds, remember that each bond (regardless of type) counts as just one electron domain. The linear shape is the only possible geometry with two electron domains.
With three electron domains, we get two possible molecular geometries:
Trigonal planar (if all domains are bonds)
Bent or angular (if one domain is a lone pair)
The electron domain geometry is always trigonal planar with three domains, but the presence of a lone pair creates the bent molecular shape.
Four electron domains create the most common and important molecular shapes:
Tetrahedral (if all domains are bonds)
Trigonal pyramidal (if one domain is a lone pair)
Bent (if two domains are lone pairs)
Quick tip: Lone pairs take up more space than bonding pairs, which is why bond angles decrease when lone pairs are present.
After determining a molecule's shape, you can predict whether it's polar or nonpolar—a critical property that affects how it interacts with other molecules.
A polar molecule has an uneven distribution of charge, with one side slightly positive and the other slightly negative. This creates a dipole (like a tiny magnet).
A nonpolar molecule has an even distribution of charge with no positive or negative ends.
Polarity depends on two factors:
Even with polar bonds, a molecule can be nonpolar if it has a symmetric shape that causes the bond dipoles to cancel out.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Saryna Cadet
@sarynacad_g5eut
Molecular geometry describes the 3D arrangement of atoms in molecules, which affects everything from a molecule's polarity to its chemical reactivity. Understanding these shapes helps predict how molecules behave and interact with each other.

Access to all documents
Improve your grades
Join milions of students
Ever wonder why some molecules can dissolve in water while others can't? It all comes down to their shape! Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule.
The shape of a molecule isn't just interesting to look at—it directly influences the molecule's properties like polarity, reactivity, and even what state of matter it exists in at room temperature.
Did you know? Carbon dioxide (CO₂) is linear, while water (H₂O) is bent. These different shapes explain why water is a liquid at room temperature while CO₂ is a gas!

Access to all documents
Improve your grades
Join milions of students
The Lewis structure of a molecule (those dot diagrams you've drawn) doesn't actually tell you how a molecule is arranged in 3D space. The real arrangement is often surprising!
For example, methane (CH₄) might look like a flat cross on paper, but in reality, the hydrogen atoms arrange themselves in a 3D tetrahedral shape with 109.5° angles between bonds. This happens because electrons naturally repel each other and seek to be as far apart as possible.
To predict these 3D arrangements, we use a model called VSEPR (Valence Shell Electron Pair Repulsion). The basic idea is simple: electron pairs around an atom, whether they're bonding or non-bonding, push away from each other as much as possible.

Access to all documents
Improve your grades
Join milions of students
Molecules can take on several common shapes depending on how many atoms and lone pairs surround the central atom:
These shapes aren't random—they're the result of electrons pushing away from each other to minimize repulsion. VSEPR theory helps us predict which shape will form in each case.

Access to all documents
Improve your grades
Join milions of students
To figure out a molecule's shape, you need to identify its electron domains—regions around the central atom where electrons are concentrated.
An electron domain can be:
Important: All bonds (single, double, or triple) count as just ONE electron domain. This is key for correctly determining geometry.
For example, carbon dioxide (CO₂) has only two electron domains (the two double bonds), making it linear. Water (H₂O) has four electron domains (two bonds and two lone pairs), making it bent.

Access to all documents
Improve your grades
Join milions of students
There are two related but different concepts you need to understand:
Electron Domain Geometry (EDG) refers to the arrangement of all electron domains (bonds and lone pairs) around the central atom. It depends only on the total number of electron domains.
Molecular Geometry refers to the actual shape formed by the atoms (not including lone pairs). It depends on both the electron domain geometry and how many of those domains are lone pairs.
For example, ammonia (NH₃) has 4 electron domains (tetrahedral EDG), but since one domain is a lone pair, its molecular geometry is trigonal pyramidal.
Remember: Lone pairs take up space but aren't visible in the final shape!

Access to all documents
Improve your grades
Join milions of students
To predict a molecule's shape:
The number of electron domains tells you the basic arrangement:
Bold tip: Remember that the molecular geometry often differs from the electron domain geometry when lone pairs are present!

Access to all documents
Improve your grades
Join milions of students
When a central atom has only two electron domains, the geometry is always linear with a bond angle of 180°.
Examples include:
In these molecules, the two electron domains push as far away from each other as possible, creating a straight line. This arrangement minimizes repulsion between the electron clouds.
Even if these molecules have double or triple bonds, remember that each bond (regardless of type) counts as just one electron domain. The linear shape is the only possible geometry with two electron domains.

Access to all documents
Improve your grades
Join milions of students
With three electron domains, we get two possible molecular geometries:
Trigonal planar (if all domains are bonds)
Bent or angular (if one domain is a lone pair)
The electron domain geometry is always trigonal planar with three domains, but the presence of a lone pair creates the bent molecular shape.

Access to all documents
Improve your grades
Join milions of students
Four electron domains create the most common and important molecular shapes:
Tetrahedral (if all domains are bonds)
Trigonal pyramidal (if one domain is a lone pair)
Bent (if two domains are lone pairs)
Quick tip: Lone pairs take up more space than bonding pairs, which is why bond angles decrease when lone pairs are present.

Access to all documents
Improve your grades
Join milions of students
After determining a molecule's shape, you can predict whether it's polar or nonpolar—a critical property that affects how it interacts with other molecules.
A polar molecule has an uneven distribution of charge, with one side slightly positive and the other slightly negative. This creates a dipole (like a tiny magnet).
A nonpolar molecule has an even distribution of charge with no positive or negative ends.
Polarity depends on two factors:
Even with polar bonds, a molecule can be nonpolar if it has a symmetric shape that causes the bond dipoles to cancel out.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
0
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user