Dive into the fascinating world of atoms and the periodic... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
69
•
Feb 1, 2026
•
UB
@uhabib
Dive into the fascinating world of atoms and the periodic... Show more






Ever wonder how scientists organized all the chemical elements? In the 19th century, Russian chemist Dmitri Mendeleev tackled this challenge by arranging about 60 known elements according to their atomic mass. His genius? He noticed patterns and left gaps where he predicted undiscovered elements should exist.
Mendeleev's predictions were so accurate that when a French scientist later claimed to discover one of these missing elements, Mendeleev correctly argued that the scientist's measurements were wrong. Talk about confidence in your work! His contributions were so significant that element 101 was named Mendelevium (Md) in his honor.
Science Spotlight: Mendeleev created his periodic table before the discovery of protons and electrons. He organized elements purely based on observed properties, making his accurate predictions even more remarkable!
The early periodic table had problems though - some elements seemed out of order, and there were mysterious gaps. These issues wouldn't be resolved until scientists gained a deeper understanding of atomic structure.
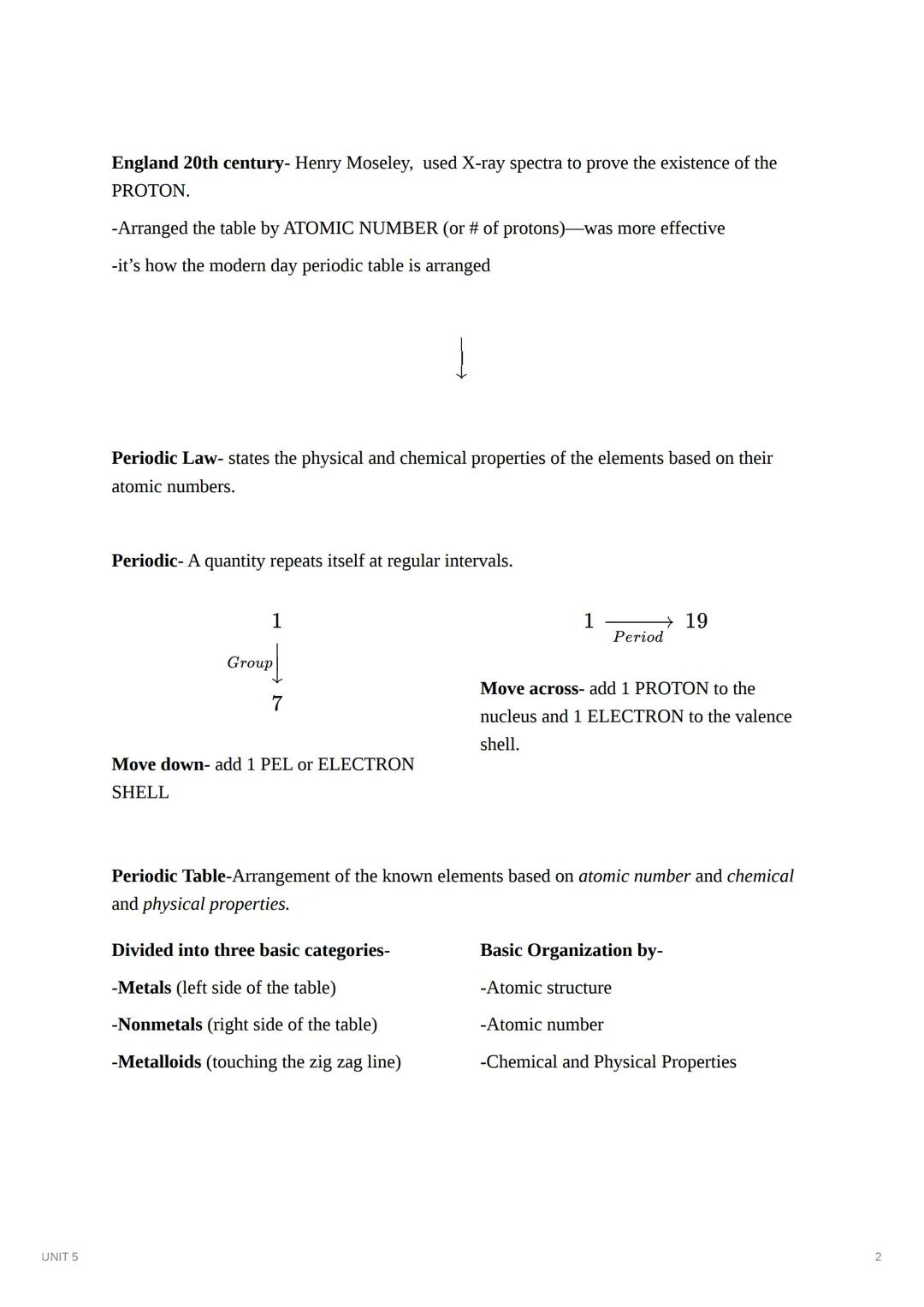
Fast forward to the 20th century: English scientist Henry Moseley used X-ray spectra to prove the existence of protons. This breakthrough led to arranging elements by atomic number (number of protons) instead of mass, creating the modern periodic table we use today.
The periodic law tells us that elements' physical and chemical properties repeat at regular intervals based on their atomic numbers. This organization creates the table's distinctive pattern:
The periodic table organizes elements into three basic categories:
Remember This: The periodic table isn't just a random arrangement - it's organized by atomic structure and shows relationships between elements' properties. This makes it a powerful tool for predicting how elements will behave!
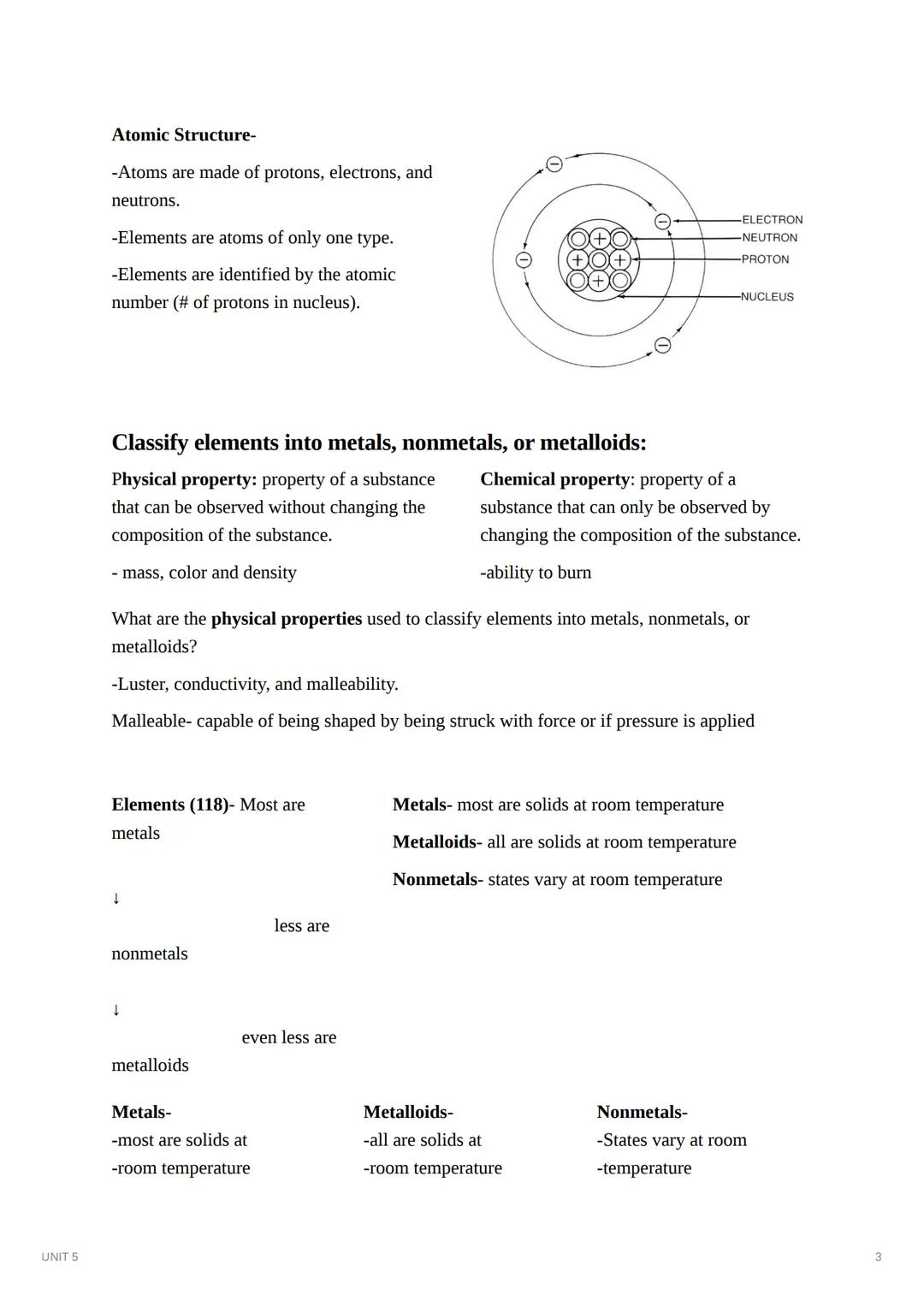
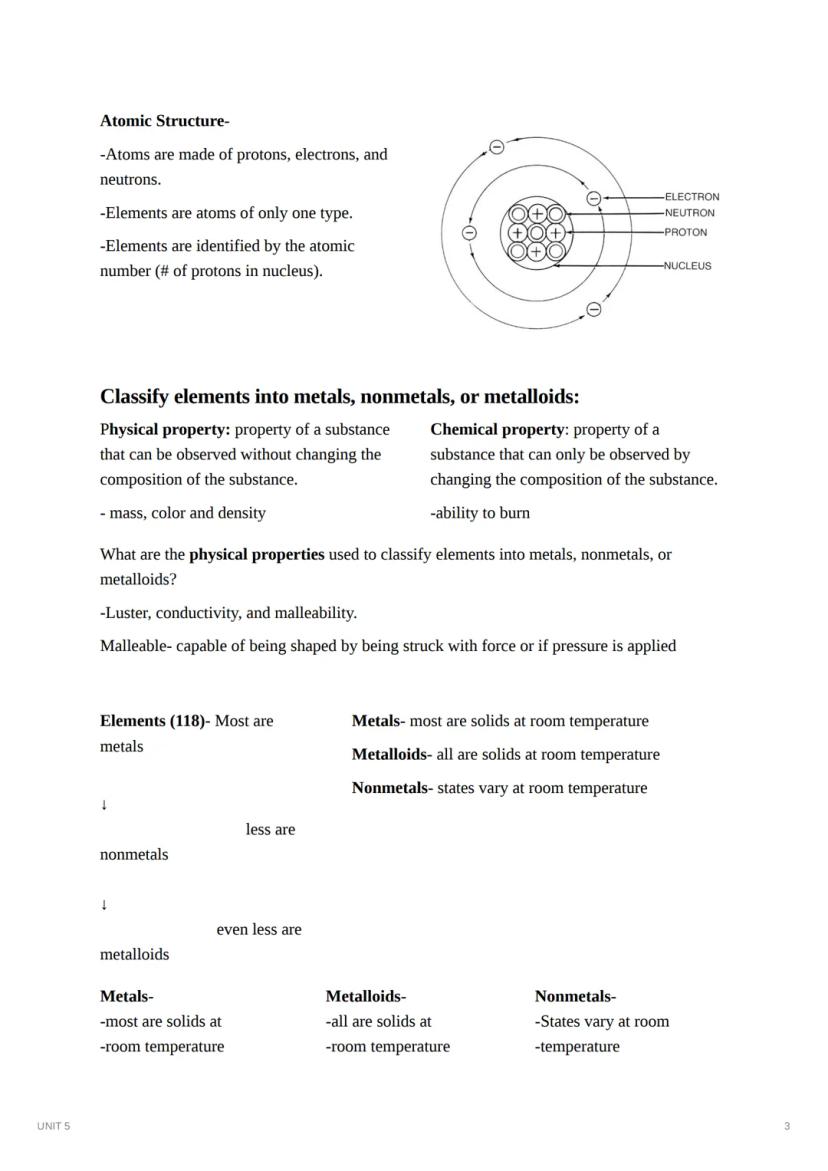
Atoms are the building blocks of matter, made up of protons, neutrons, and electrons. Each element consists of atoms with the same number of protons (atomic number), giving that element its unique identity. The nucleus contains protons and neutrons, while electrons orbit around it.
Scientists classify elements as metals, nonmetals, or metalloids based on their physical properties like luster, conductivity, and malleability. Malleability refers to a material's ability to be shaped when force or pressure is applied. Most elements are metals, fewer are nonmetals, and even fewer are metalloids.
Each category has distinctive characteristics:
Cool Chemistry Fact: At standard temperature and pressure (STP), most elements are solids. Only hydrogen, nitrogen, oxygen, fluorine, chlorine, and the noble gases are gases, while just two elements are naturally liquid: mercury (a metal) and bromine (a nonmetal).

Some elements don't exist naturally as single atoms but pair up as diatomic elements. Remember "Have No Fear Of Ice Cold Bears" (H₂, N₂, O₂, F₂, Cl₂, Br₂, I₂) to recall these seven elements that naturally form two-atom molecules for stability.
Allotropy is when an element exists in different forms in the same phase. Carbon demonstrates this beautifully as diamond, graphite, and coal - same element, dramatically different properties! Oxygen exists as both O₂ (what we breathe) and O₃ (ozone).
The concept of atoms has evolved dramatically over time. Ancient Greek philosophers like Democritus first proposed the idea of indivisible particles called "atomos" around 460-370 BCE, but without evidence, few believed him. Aristotle's competing theory claimed all substances were made of earth, fire, air, and water.
John Dalton revolutionized chemistry in the 1800s with his atomic theory, proposing that:
Historical Perspective: Dalton's "cannonball theory" visualized atoms as solid, uniform spheres - quite different from our modern understanding! While simple, this model was a huge leap forward for chemistry.
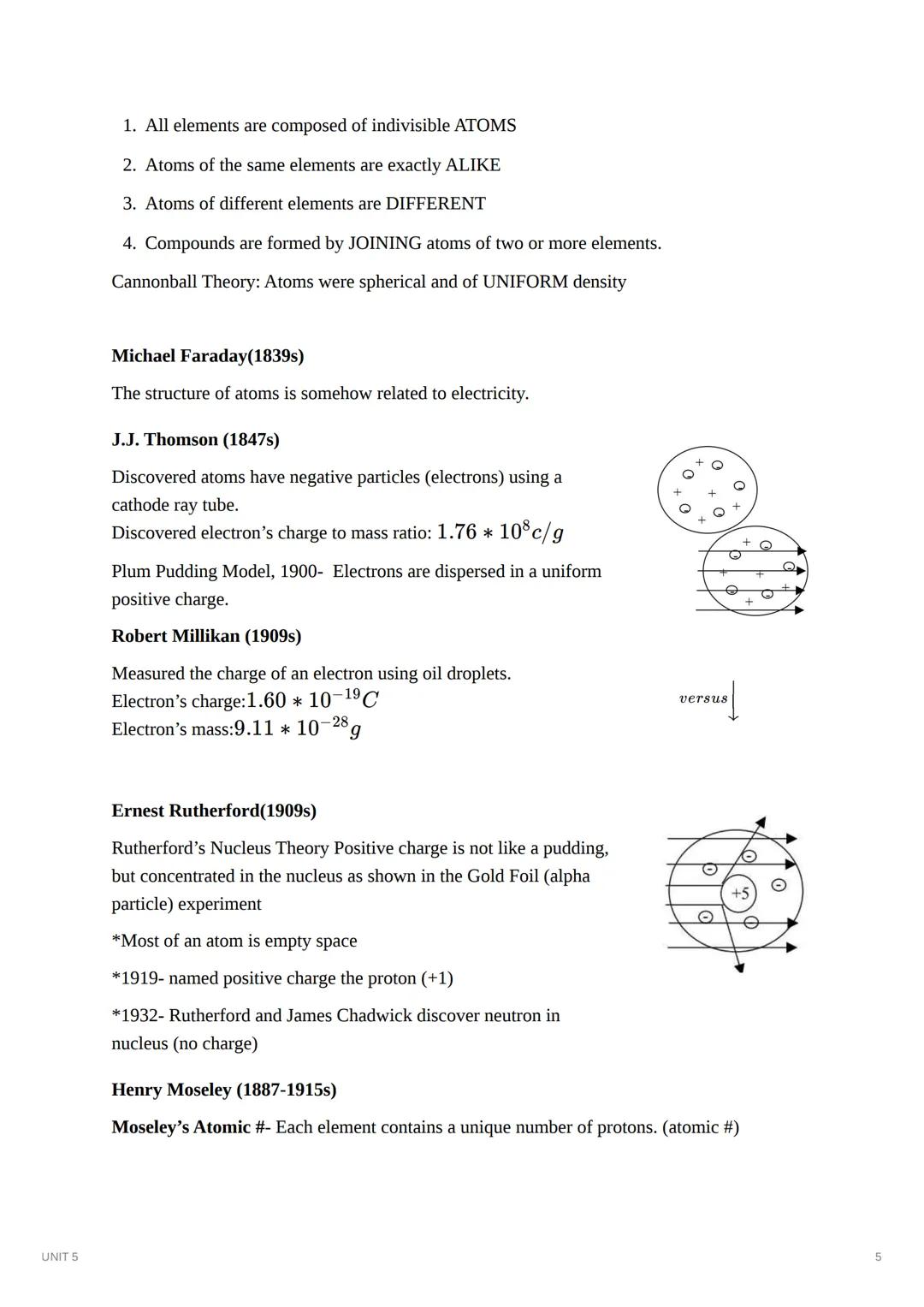
The atomic model evolved rapidly in the late 19th and early 20th centuries as scientists made groundbreaking discoveries. Michael Faraday first connected atoms to electricity in the 1830s, setting the stage for further exploration.
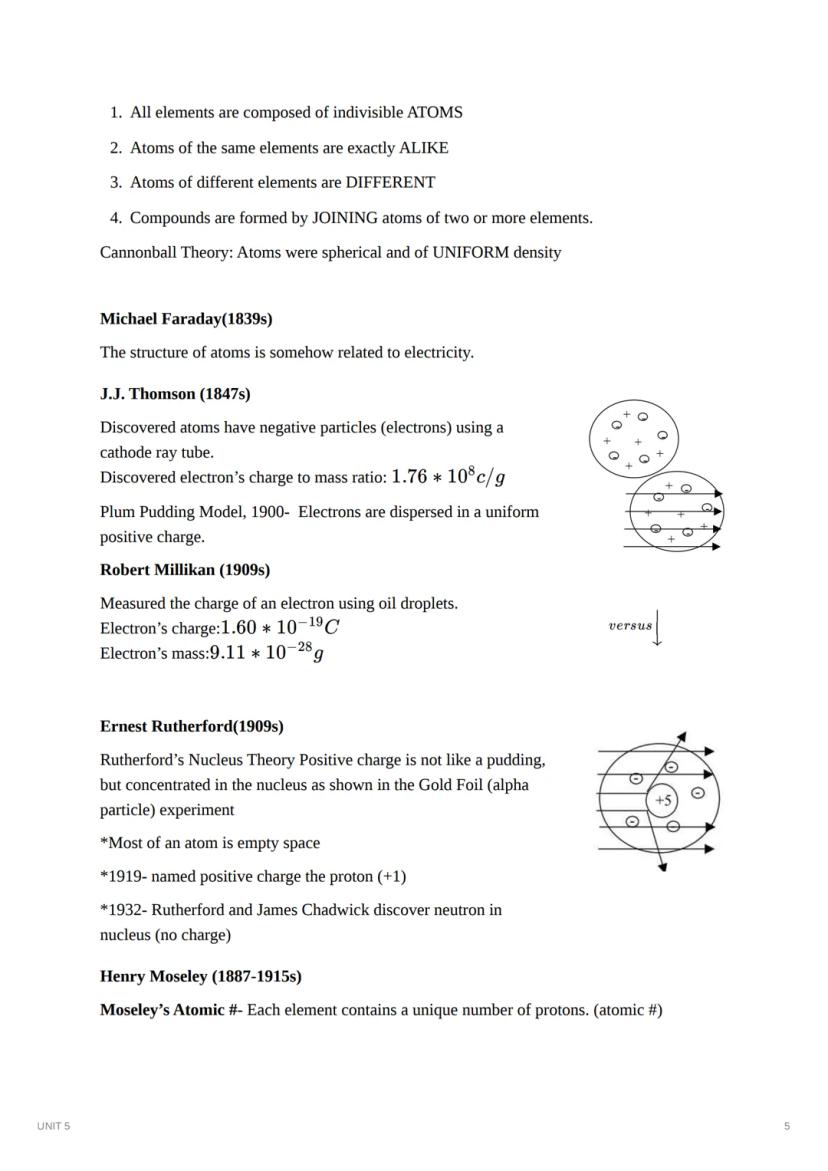
J.J. Thomson discovered negatively charged particles (electrons) in 1897 using a cathode ray tube. His "plum pudding model" imagined electrons embedded within a positively charged substance, similar to raisins in pudding. Robert Millikan later measured the precise charge of an electron through his famous oil drop experiment.
Ernest Rutherford transformed our understanding of atomic structure in 1909 with his gold foil experiment. When alpha particles passed through gold foil, most went straight through, but some bounced back dramatically. This proved that atoms contain a dense, positively charged nucleus surrounded mostly by empty space. Rutherford named the positive particles "protons" and later, with James Chadwick, discovered neutrons.
Think About It: Rutherford's discovery that atoms are mostly empty space with a tiny, dense nucleus completely contradicted Thomson's model. This shows how science advances by challenging existing theories with new evidence!
Henry Moseley's work established that each element contains a unique number of protons - what we now call the atomic number - which became the basis for organizing the periodic table.
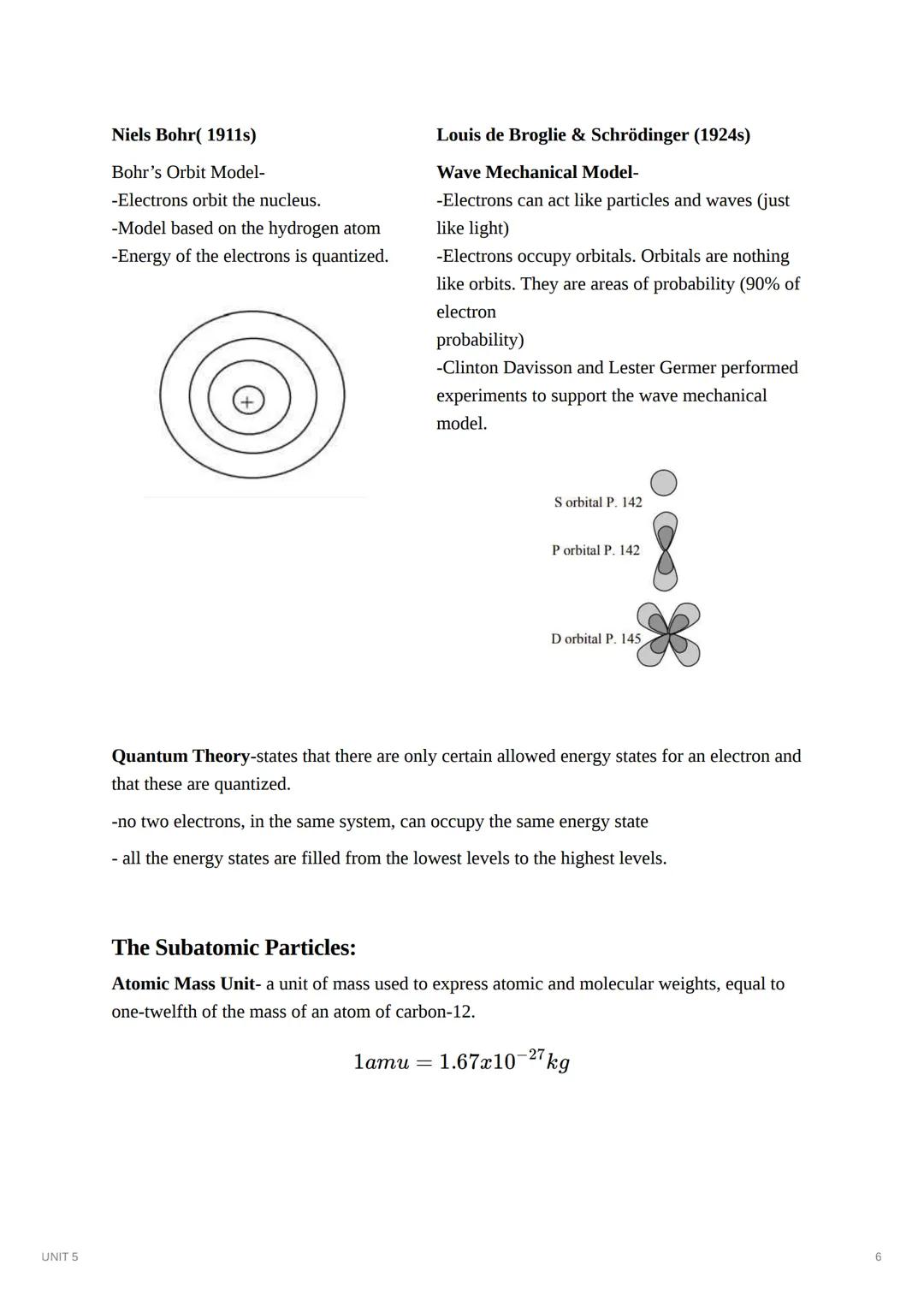
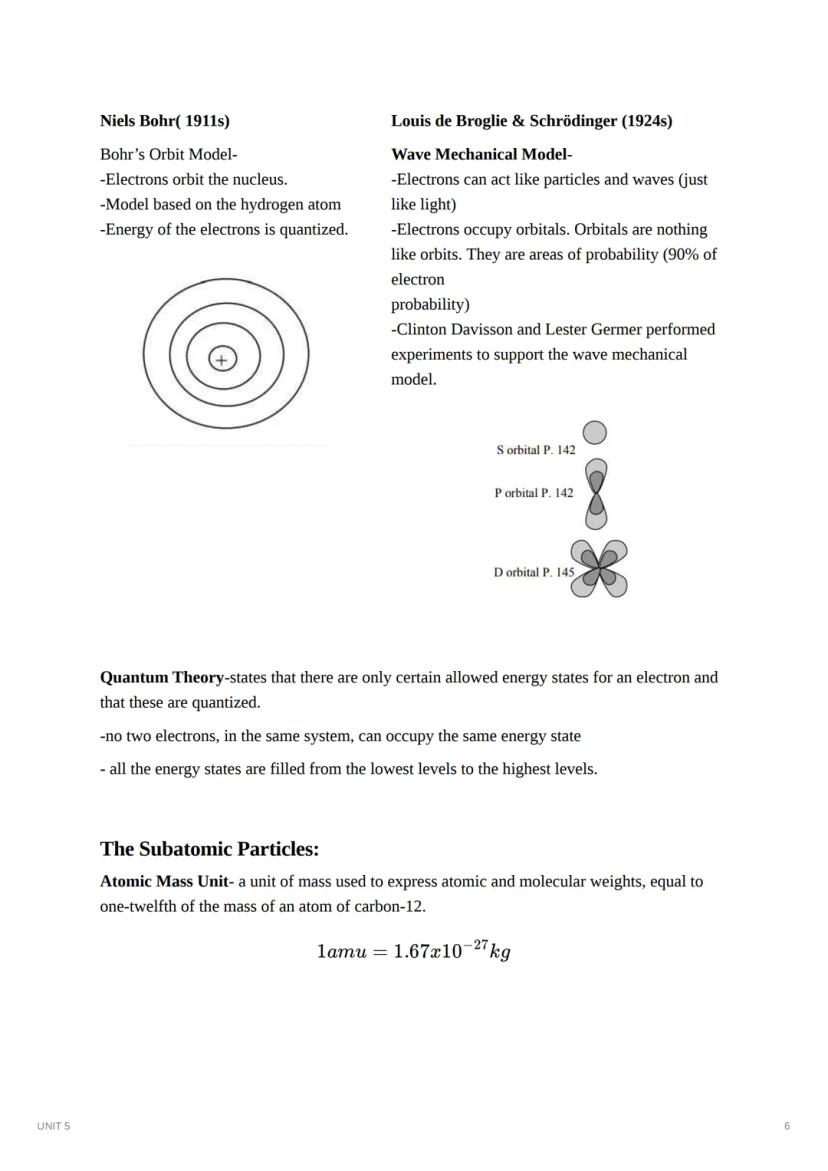
Niels Bohr revolutionized atomic theory in 1911 by proposing that electrons orbit the nucleus in specific energy levels or "shells." His model explained why atoms emit specific colors of light when heated - electrons jump between these quantized energy levels.
By the 1920s, scientists Louis de Broglie and Erwin Schrödinger developed the wave mechanical model, showing that electrons behave as both particles and waves. Instead of defined orbits, electrons exist in "orbitals" - regions of probability where electrons are likely to be found. This model gives us the s, p, d, and f orbitals that determine an element's properties.
Quantum theory states that electrons can only exist at specific energy states (they're "quantized"), no two electrons in the same system can occupy the same energy state, and energy states fill from lowest to highest levels. This explains electron configurations and chemical behavior.
Make It Concrete: Think of electron orbitals like a 3D probability map. An s orbital is spherical (like a ball around the nucleus), while p orbitals are dumbbell-shaped and point in different directions. These shapes determine how atoms bond!
The three main subatomic particles have different properties:
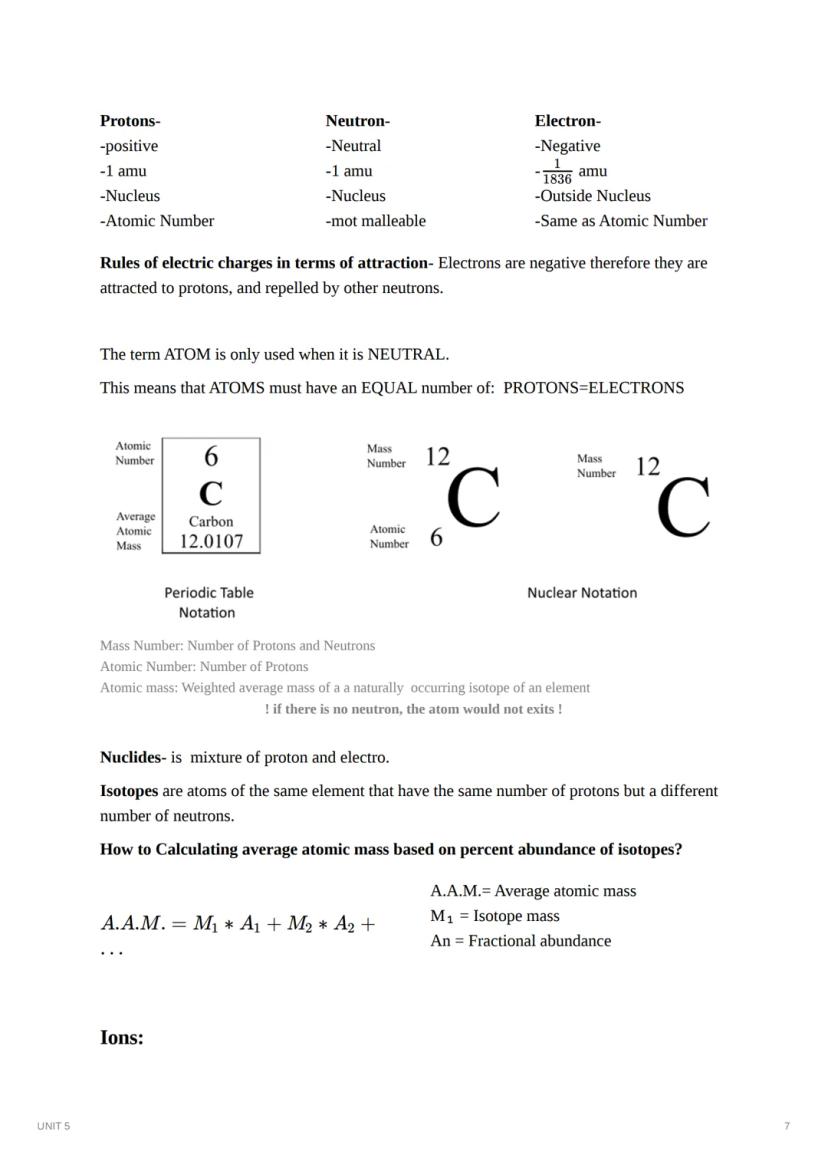
The term "atom" only applies when the particle is neutral - meaning it has an equal number of protons and electrons. This balance creates a particle with no overall charge, which is how elements normally exist.
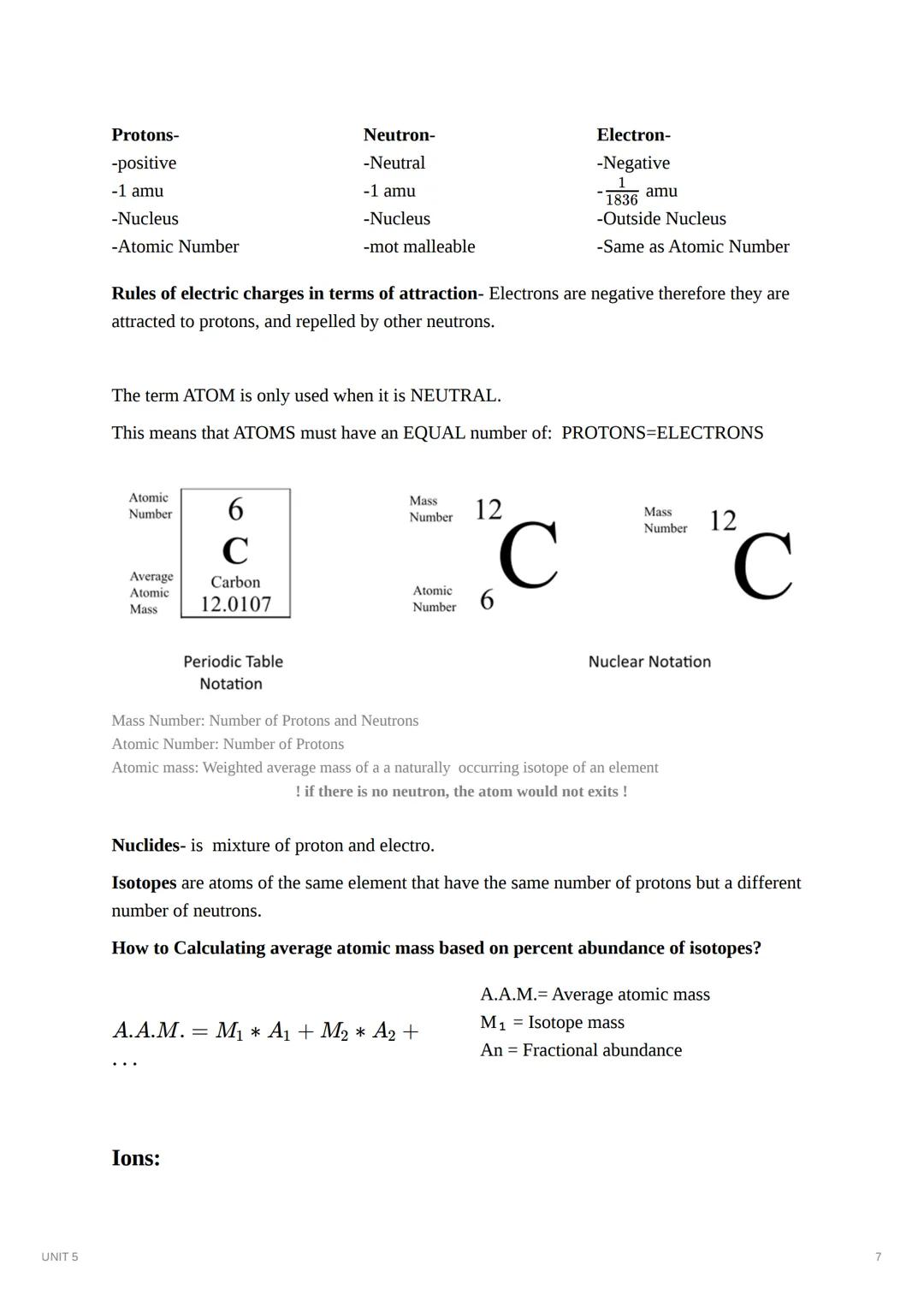
On the periodic table, each element has several important numbers:
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. For example, carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons but 8 neutrons. The atomic mass listed on the periodic table is actually an average based on the natural abundance of each isotope.
You can calculate the average atomic mass using this formula: Average atomic mass = (Mass₁ × Abundance₁) + (Mass₂ × Abundance₂) + ...
Why It Matters: Isotopes have identical chemical properties but different physical properties. Some isotopes are stable, while others are radioactive. Carbon-14, for example, is used in carbon dating to determine the age of ancient objects!
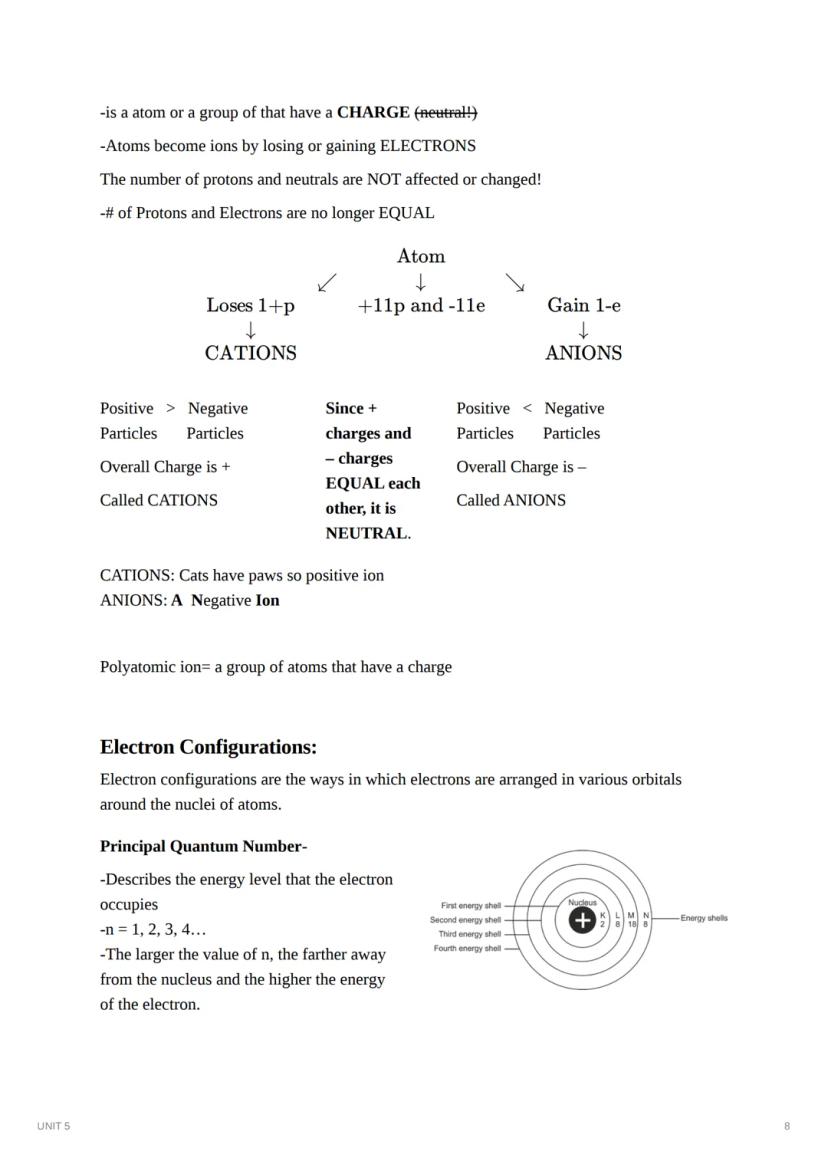
Ions form when atoms gain or lose electrons, creating charged particles. Unlike isotopes, ions have different chemical properties than their parent atoms because their electron count has changed.
Ions form when atoms gain or lose electrons while keeping their proton and neutron counts the same. When an atom loses electrons, it forms a cation with a positive charge (fewer electrons than protons). When an atom gains electrons, it forms an anion with a negative charge (more electrons than protons).
A fun way to remember: CATIONS have a "paws-itive" charge (like cats), while ANIONS are "A Negative ION." Multiple atoms bonded together with a charge form polyatomic ions.
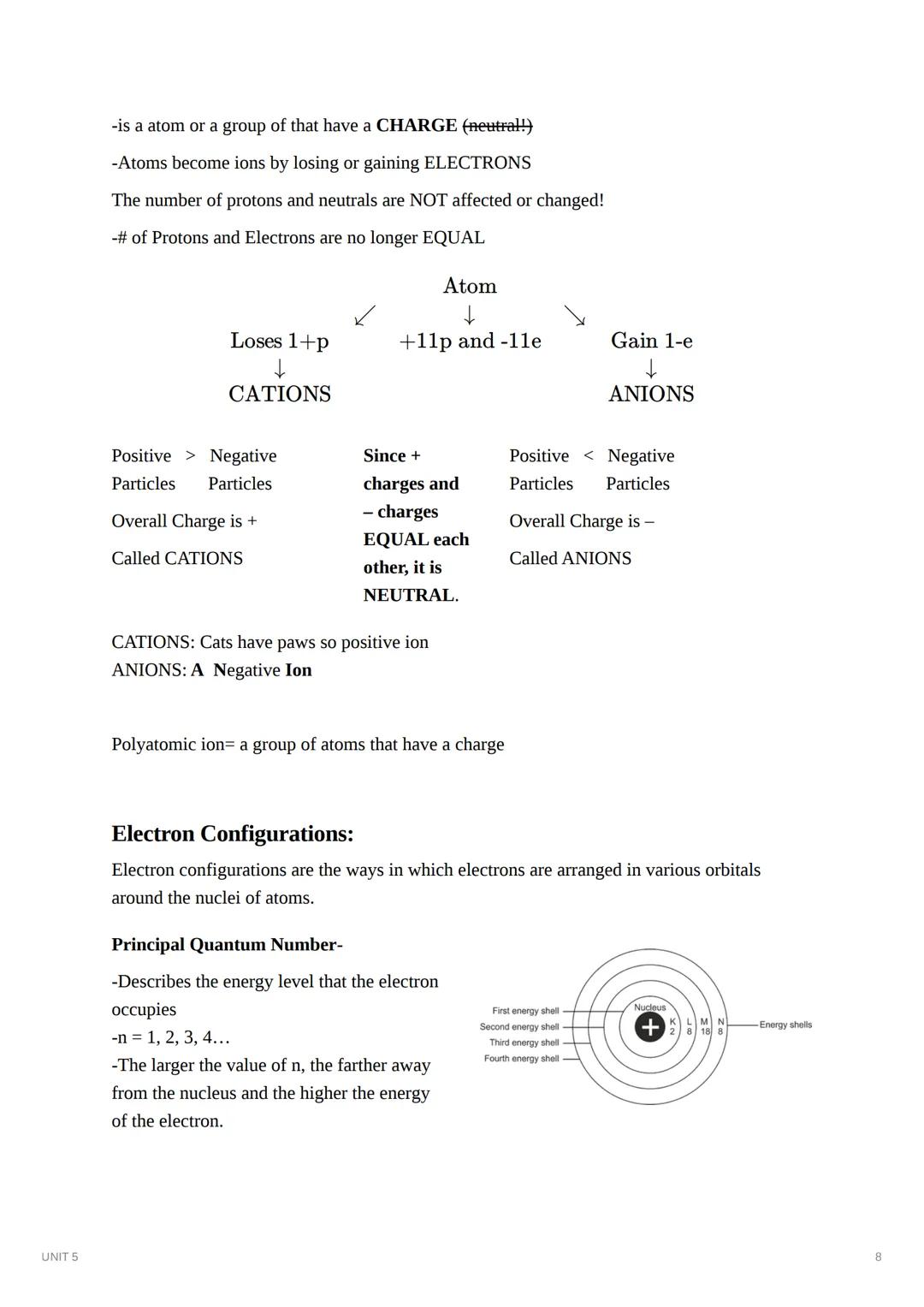
Electron configurations describe how electrons are arranged in orbitals around an atom's nucleus. Each electron occupies a specific energy level and sublevel. The principal quantum number (n) indicates the energy level (1, 2, 3, 4...), with higher numbers representing higher energy and greater distance from the nucleus.
Each energy level contains sublevels labeled s, p, d, and f. The number of sublevels equals the principal quantum number. For example:
Chemistry Hack: You can quickly determine the maximum number of electrons in each energy level using the formula 2n², where n is the principal quantum number. So level 1 holds 2 electrons, level 2 holds 8, level 3 holds 18, and level 4 holds 32!

Valence electrons are the electrons in an atom's outermost shell and determine its chemical properties. These electrons participate in bonding and can be visually represented using electron dot diagrams (also called Lewis structures).
To create an electron dot diagram:
The octet rule explains why atoms form chemical bonds - they tend to gain, lose, or share electrons to achieve eight electrons in their valence shell, which is the stable configuration found in noble gases. Exceptions include hydrogen and helium, which follow the duet rule, needing only two electrons to be stable.
Elements in the first and second periods like hydrogen, helium, lithium, beryllium, and boron are exceptions to the octet rule as they can be stable with fewer than eight valence electrons.
Think Deeper: Why do atoms "want" eight valence electrons? This configuration mimics the stable electron arrangement of noble gases, which rarely react with other elements because their valence shells are already filled!
The ground state of an atom is its lowest energy state, where all electrons occupy the lowest possible energy levels. When an atom absorbs energy, electrons jump to higher energy levels, creating an excited state.

When an atom transitions from ground state to excited state, it absorbs energy. When returning from excited to ground state, it releases energy as light. This released light creates a unique bright-line spectrum for each element - like a chemical fingerprint that scientists use to identify elements.
You can identify elements in a mixture by comparing their bright-line spectra. If every line from an element's spectrum appears in the mixture's spectrum, that element is present in the mixture.
The difference between ground state and excited state configurations is the arrangement of electrons. For example:
Atomic radius refers to half the distance between two neighboring nuclei of the same element in solid form. As you move through the periodic table, atomic radius follows clear patterns:
Make Connections: The patterns in atomic radius help explain chemical reactivity. Elements with larger atomic radii tend to lose electrons more easily, while those with smaller radii tend to gain electrons. This directly impacts how elements form bonds!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
UB
@uhabib
Dive into the fascinating world of atoms and the periodic table! This unit explores how elements are organized, the structure of atoms, and how electron arrangements affect chemical properties. You'll discover the key scientists who shaped our understanding of atomic... Show more

Access to all documents
Improve your grades
Join milions of students
Ever wonder how scientists organized all the chemical elements? In the 19th century, Russian chemist Dmitri Mendeleev tackled this challenge by arranging about 60 known elements according to their atomic mass. His genius? He noticed patterns and left gaps where he predicted undiscovered elements should exist.
Mendeleev's predictions were so accurate that when a French scientist later claimed to discover one of these missing elements, Mendeleev correctly argued that the scientist's measurements were wrong. Talk about confidence in your work! His contributions were so significant that element 101 was named Mendelevium (Md) in his honor.
Science Spotlight: Mendeleev created his periodic table before the discovery of protons and electrons. He organized elements purely based on observed properties, making his accurate predictions even more remarkable!
The early periodic table had problems though - some elements seemed out of order, and there were mysterious gaps. These issues wouldn't be resolved until scientists gained a deeper understanding of atomic structure.

Access to all documents
Improve your grades
Join milions of students
Fast forward to the 20th century: English scientist Henry Moseley used X-ray spectra to prove the existence of protons. This breakthrough led to arranging elements by atomic number (number of protons) instead of mass, creating the modern periodic table we use today.
The periodic law tells us that elements' physical and chemical properties repeat at regular intervals based on their atomic numbers. This organization creates the table's distinctive pattern:
The periodic table organizes elements into three basic categories:
Remember This: The periodic table isn't just a random arrangement - it's organized by atomic structure and shows relationships between elements' properties. This makes it a powerful tool for predicting how elements will behave!

Access to all documents
Improve your grades
Join milions of students
Atoms are the building blocks of matter, made up of protons, neutrons, and electrons. Each element consists of atoms with the same number of protons (atomic number), giving that element its unique identity. The nucleus contains protons and neutrons, while electrons orbit around it.
Scientists classify elements as metals, nonmetals, or metalloids based on their physical properties like luster, conductivity, and malleability. Malleability refers to a material's ability to be shaped when force or pressure is applied. Most elements are metals, fewer are nonmetals, and even fewer are metalloids.
Each category has distinctive characteristics:
Cool Chemistry Fact: At standard temperature and pressure (STP), most elements are solids. Only hydrogen, nitrogen, oxygen, fluorine, chlorine, and the noble gases are gases, while just two elements are naturally liquid: mercury (a metal) and bromine (a nonmetal).

Access to all documents
Improve your grades
Join milions of students
Some elements don't exist naturally as single atoms but pair up as diatomic elements. Remember "Have No Fear Of Ice Cold Bears" (H₂, N₂, O₂, F₂, Cl₂, Br₂, I₂) to recall these seven elements that naturally form two-atom molecules for stability.
Allotropy is when an element exists in different forms in the same phase. Carbon demonstrates this beautifully as diamond, graphite, and coal - same element, dramatically different properties! Oxygen exists as both O₂ (what we breathe) and O₃ (ozone).
The concept of atoms has evolved dramatically over time. Ancient Greek philosophers like Democritus first proposed the idea of indivisible particles called "atomos" around 460-370 BCE, but without evidence, few believed him. Aristotle's competing theory claimed all substances were made of earth, fire, air, and water.
John Dalton revolutionized chemistry in the 1800s with his atomic theory, proposing that:
Historical Perspective: Dalton's "cannonball theory" visualized atoms as solid, uniform spheres - quite different from our modern understanding! While simple, this model was a huge leap forward for chemistry.

Access to all documents
Improve your grades
Join milions of students
The atomic model evolved rapidly in the late 19th and early 20th centuries as scientists made groundbreaking discoveries. Michael Faraday first connected atoms to electricity in the 1830s, setting the stage for further exploration.
J.J. Thomson discovered negatively charged particles (electrons) in 1897 using a cathode ray tube. His "plum pudding model" imagined electrons embedded within a positively charged substance, similar to raisins in pudding. Robert Millikan later measured the precise charge of an electron through his famous oil drop experiment.
Ernest Rutherford transformed our understanding of atomic structure in 1909 with his gold foil experiment. When alpha particles passed through gold foil, most went straight through, but some bounced back dramatically. This proved that atoms contain a dense, positively charged nucleus surrounded mostly by empty space. Rutherford named the positive particles "protons" and later, with James Chadwick, discovered neutrons.
Think About It: Rutherford's discovery that atoms are mostly empty space with a tiny, dense nucleus completely contradicted Thomson's model. This shows how science advances by challenging existing theories with new evidence!
Henry Moseley's work established that each element contains a unique number of protons - what we now call the atomic number - which became the basis for organizing the periodic table.

Access to all documents
Improve your grades
Join milions of students
Niels Bohr revolutionized atomic theory in 1911 by proposing that electrons orbit the nucleus in specific energy levels or "shells." His model explained why atoms emit specific colors of light when heated - electrons jump between these quantized energy levels.
By the 1920s, scientists Louis de Broglie and Erwin Schrödinger developed the wave mechanical model, showing that electrons behave as both particles and waves. Instead of defined orbits, electrons exist in "orbitals" - regions of probability where electrons are likely to be found. This model gives us the s, p, d, and f orbitals that determine an element's properties.
Quantum theory states that electrons can only exist at specific energy states (they're "quantized"), no two electrons in the same system can occupy the same energy state, and energy states fill from lowest to highest levels. This explains electron configurations and chemical behavior.
Make It Concrete: Think of electron orbitals like a 3D probability map. An s orbital is spherical (like a ball around the nucleus), while p orbitals are dumbbell-shaped and point in different directions. These shapes determine how atoms bond!
The three main subatomic particles have different properties:

Access to all documents
Improve your grades
Join milions of students
The term "atom" only applies when the particle is neutral - meaning it has an equal number of protons and electrons. This balance creates a particle with no overall charge, which is how elements normally exist.
On the periodic table, each element has several important numbers:
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. For example, carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons but 8 neutrons. The atomic mass listed on the periodic table is actually an average based on the natural abundance of each isotope.
You can calculate the average atomic mass using this formula: Average atomic mass = (Mass₁ × Abundance₁) + (Mass₂ × Abundance₂) + ...
Why It Matters: Isotopes have identical chemical properties but different physical properties. Some isotopes are stable, while others are radioactive. Carbon-14, for example, is used in carbon dating to determine the age of ancient objects!
Ions form when atoms gain or lose electrons, creating charged particles. Unlike isotopes, ions have different chemical properties than their parent atoms because their electron count has changed.

Access to all documents
Improve your grades
Join milions of students
Ions form when atoms gain or lose electrons while keeping their proton and neutron counts the same. When an atom loses electrons, it forms a cation with a positive charge (fewer electrons than protons). When an atom gains electrons, it forms an anion with a negative charge (more electrons than protons).
A fun way to remember: CATIONS have a "paws-itive" charge (like cats), while ANIONS are "A Negative ION." Multiple atoms bonded together with a charge form polyatomic ions.
Electron configurations describe how electrons are arranged in orbitals around an atom's nucleus. Each electron occupies a specific energy level and sublevel. The principal quantum number (n) indicates the energy level (1, 2, 3, 4...), with higher numbers representing higher energy and greater distance from the nucleus.
Each energy level contains sublevels labeled s, p, d, and f. The number of sublevels equals the principal quantum number. For example:
Chemistry Hack: You can quickly determine the maximum number of electrons in each energy level using the formula 2n², where n is the principal quantum number. So level 1 holds 2 electrons, level 2 holds 8, level 3 holds 18, and level 4 holds 32!

Access to all documents
Improve your grades
Join milions of students
Valence electrons are the electrons in an atom's outermost shell and determine its chemical properties. These electrons participate in bonding and can be visually represented using electron dot diagrams (also called Lewis structures).
To create an electron dot diagram:
The octet rule explains why atoms form chemical bonds - they tend to gain, lose, or share electrons to achieve eight electrons in their valence shell, which is the stable configuration found in noble gases. Exceptions include hydrogen and helium, which follow the duet rule, needing only two electrons to be stable.
Elements in the first and second periods like hydrogen, helium, lithium, beryllium, and boron are exceptions to the octet rule as they can be stable with fewer than eight valence electrons.
Think Deeper: Why do atoms "want" eight valence electrons? This configuration mimics the stable electron arrangement of noble gases, which rarely react with other elements because their valence shells are already filled!
The ground state of an atom is its lowest energy state, where all electrons occupy the lowest possible energy levels. When an atom absorbs energy, electrons jump to higher energy levels, creating an excited state.

Access to all documents
Improve your grades
Join milions of students
When an atom transitions from ground state to excited state, it absorbs energy. When returning from excited to ground state, it releases energy as light. This released light creates a unique bright-line spectrum for each element - like a chemical fingerprint that scientists use to identify elements.
You can identify elements in a mixture by comparing their bright-line spectra. If every line from an element's spectrum appears in the mixture's spectrum, that element is present in the mixture.
The difference between ground state and excited state configurations is the arrangement of electrons. For example:
Atomic radius refers to half the distance between two neighboring nuclei of the same element in solid form. As you move through the periodic table, atomic radius follows clear patterns:
Make Connections: The patterns in atomic radius help explain chemical reactivity. Elements with larger atomic radii tend to lose electrons more easily, while those with smaller radii tend to gain electrons. This directly impacts how elements form bonds!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
1
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
ionic and covalent compounds
percentage composition of a compound
Explore the concept of isotopes in chemistry, focusing on atomic number, mass number, and the differences in neutron count. This summary provides a clear definition and examples to enhance your understanding of isotopes and their significance in the study of elements.
This note contains the summary of the topics redox reaction, electrochemistry, nuclear chemistry, and stoichiometry.
An introduction to chemistry including the different branches of chemistry.
Learn about the reactivity and properties of metals, alkali metals, alkaline earth metals, and transition metals in the periodic table.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user