Ready to dive into the world of atoms, molecules, and... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

9
0
Liwayway
12/14/2025
GenChem
General Chemistry 1
747
•
Dec 14, 2025
•
Liwayway
@liwayway
Ready to dive into the world of atoms, molecules, and... Show more





Ever wondered what everything around you is actually made of? Atoms are the smallest particles that make up all matter, while molecules are formed when atoms team up. When these particles gain or lose electrons, they become ions with electric charges.
Matter exists in three main states based on how tightly packed particles are. Solids have particles packed closely together with restricted movement, liquids have particles farther apart with free movement, and gases have particles very far apart moving chaotically.
Understanding physical properties (like color or melting point) versus chemical properties (like flammability) is crucial for your exams. Physical properties don't change the substance's composition when measured, while chemical properties do involve composition changes.
Pure substances contain only one component (like elements such as gold or compounds like water), while mixtures combine several components. You can separate mixtures using techniques like filtration, distillation, or chromatography depending on what you're trying to separate.
Key Tip: Remember that elements contain only one type of atom, while compounds contain two or more different types of atoms bonded together.

Chemical formulas are like recipes that tell you exactly what's in a compound and in what proportions. They're essential for writing chemical equations and understanding how substances react with each other.
The molecular formula shows the actual number of each type of atom in a molecule (like C₆H₁₂O₆ for glucose). The empirical formula gives you the simplest whole-number ratio of elements (like CH₂O for glucose). Think of empirical formulas as the "reduced fraction" version of molecular formulas.
To find molecular formulas from empirical formulas, you'll need to calculate the empirical formula mass and compare it to the given molar mass. The ratio tells you what to multiply the empirical formula by to get the molecular formula.
Percent composition calculations help you determine what percentage of a compound's mass comes from each element. This is super useful for identifying unknown compounds in lab work.
Study Hack: Practice converting between molecular and empirical formulas - this concept appears frequently on tests and is the foundation for more advanced topics.

Balancing chemical equations is like solving a puzzle - you need equal numbers of each type of atom on both sides. Master this skill because it's the gateway to understanding all chemical reactions.
Mole ratios from balanced equations tell you exactly how much of each substance you need or will produce. For the reaction 2KClO₃ → 2KCl + 3O₂, the mole ratio of KClO₃ to O₂ is 2:3, meaning 2 moles of potassium chlorate produce 3 moles of oxygen.
Understanding limiting reactants helps you figure out which substance gets used up first and limits how much product you can make. The percent yield compares what you actually get in a reaction to what you theoretically should get.
Gas laws like Boyle's Law (pressure and volume are inversely related) and Gay-Lussac's Law (pressure and temperature are directly related) explain how gases behave under different conditions. These relationships are crucial for solving gas problems.
Real-World Connection: Percent yield calculations are used in pharmaceutical manufacturing to determine how efficient drug production processes are.

The ideal gas law is your ultimate tool for solving gas problems. This equation connects pressure, volume, temperature, and the amount of gas in one powerful formula that works for most real-world situations.
When working with gas mixtures, Dalton's Law states that total pressure equals the sum of all partial pressures. Partial pressure is the pressure each gas would exert if it were alone in the container.
Mole fraction helps you calculate partial pressures by showing what fraction of the total moles each gas contributes. Simply multiply the total pressure by the mole fraction to get the partial pressure of any gas in a mixture.
For gaseous reactions, you can use stoichiometry just like with other reactions. The volume ratios often match the mole ratios from the balanced equation when temperature and pressure stay constant.
Problem-Solving Tip: Always convert temperature to Kelvin and make sure your units match when using the ideal gas law - this prevents most calculation errors.
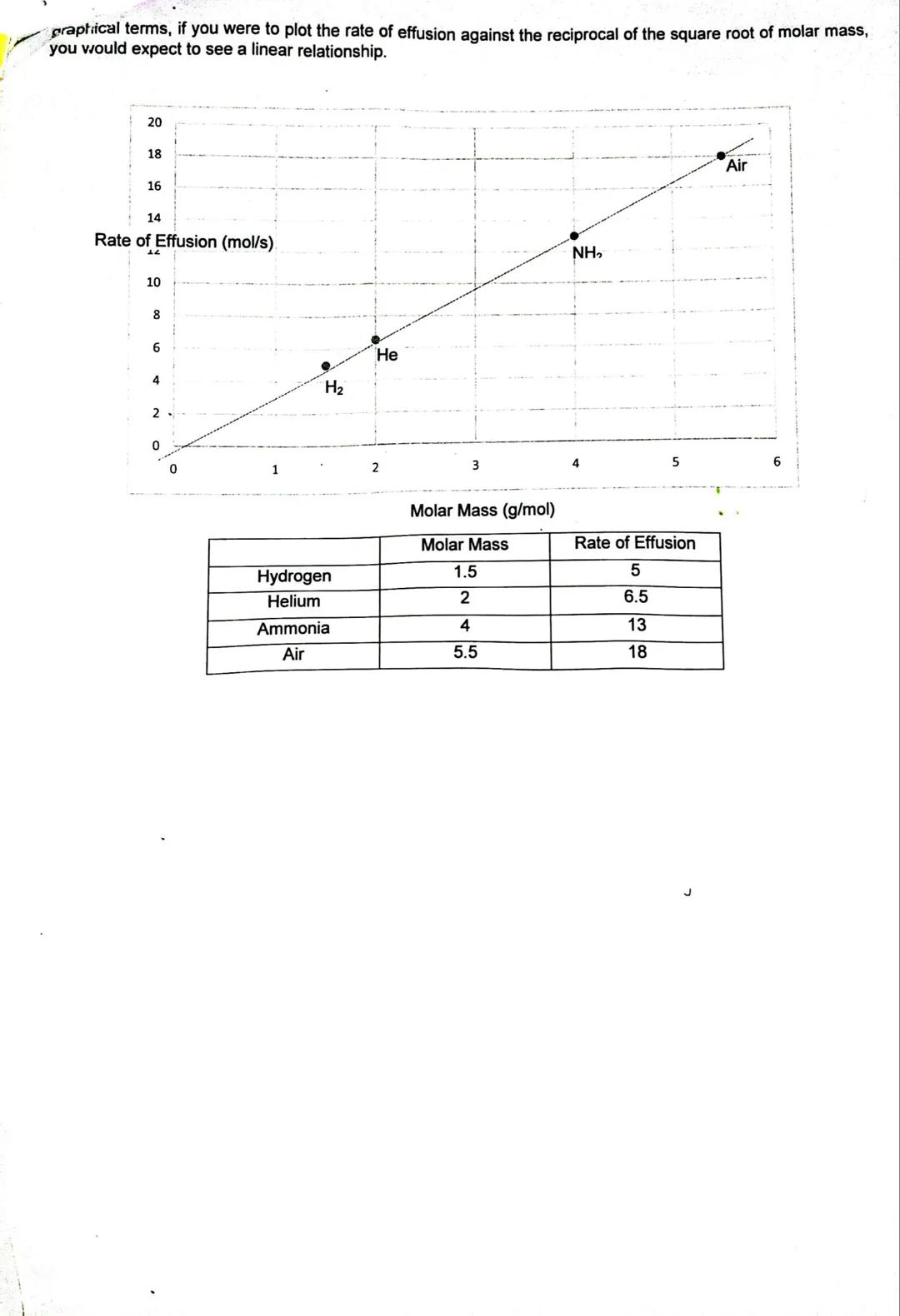
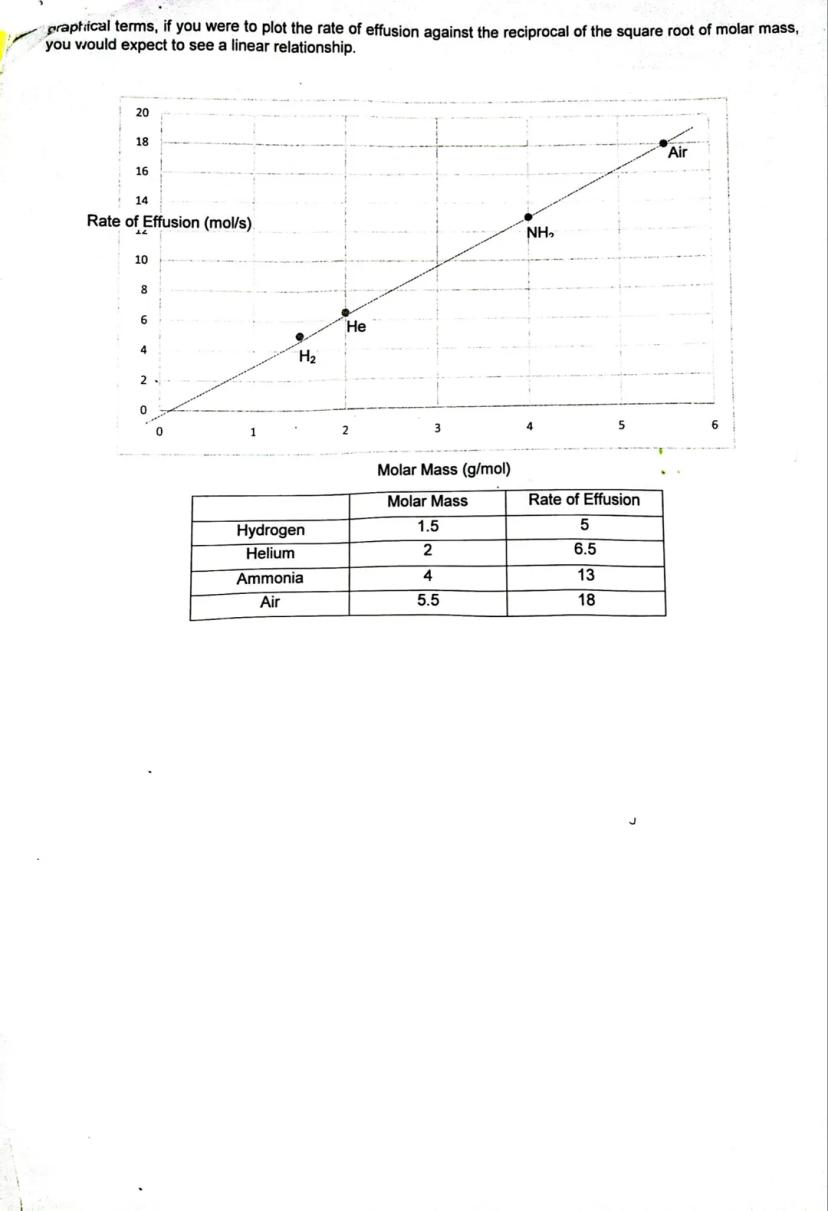
Graham's Law explains why some gases escape from containers faster than others. The rate of effusion (how fast gas particles escape through tiny holes) depends on the gas's molar mass - lighter gases move faster than heavier ones.
The mathematical relationship shows that effusion rate is inversely proportional to the square root of molar mass. This means hydrogen gas (very light) effuses much faster than ammonia gas (heavier).
You can use this principle to compare effusion rates of different gases or even identify unknown gases based on how quickly they escape. The graph shows this relationship clearly - as molar mass increases, the effusion rate decreases.
This concept connects to kinetic molecular theory, which explains that lighter gas particles move faster at the same temperature. Understanding this helps explain many real-world phenomena, like why helium balloons deflate faster than regular air-filled balloons.
Lab Application: Graham's Law is often tested through experiments comparing how fast different gases diffuse through porous materials or escape from containers.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Liwayway
@liwayway
Ready to dive into the world of atoms, molecules, and chemical reactions? General Chemistry I covers everything from the tiniest particles that make up matter to the gas laws that explain how substances behave under different conditions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered what everything around you is actually made of? Atoms are the smallest particles that make up all matter, while molecules are formed when atoms team up. When these particles gain or lose electrons, they become ions with electric charges.
Matter exists in three main states based on how tightly packed particles are. Solids have particles packed closely together with restricted movement, liquids have particles farther apart with free movement, and gases have particles very far apart moving chaotically.
Understanding physical properties (like color or melting point) versus chemical properties (like flammability) is crucial for your exams. Physical properties don't change the substance's composition when measured, while chemical properties do involve composition changes.
Pure substances contain only one component (like elements such as gold or compounds like water), while mixtures combine several components. You can separate mixtures using techniques like filtration, distillation, or chromatography depending on what you're trying to separate.
Key Tip: Remember that elements contain only one type of atom, while compounds contain two or more different types of atoms bonded together.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical formulas are like recipes that tell you exactly what's in a compound and in what proportions. They're essential for writing chemical equations and understanding how substances react with each other.
The molecular formula shows the actual number of each type of atom in a molecule (like C₆H₁₂O₆ for glucose). The empirical formula gives you the simplest whole-number ratio of elements (like CH₂O for glucose). Think of empirical formulas as the "reduced fraction" version of molecular formulas.
To find molecular formulas from empirical formulas, you'll need to calculate the empirical formula mass and compare it to the given molar mass. The ratio tells you what to multiply the empirical formula by to get the molecular formula.
Percent composition calculations help you determine what percentage of a compound's mass comes from each element. This is super useful for identifying unknown compounds in lab work.
Study Hack: Practice converting between molecular and empirical formulas - this concept appears frequently on tests and is the foundation for more advanced topics.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Balancing chemical equations is like solving a puzzle - you need equal numbers of each type of atom on both sides. Master this skill because it's the gateway to understanding all chemical reactions.
Mole ratios from balanced equations tell you exactly how much of each substance you need or will produce. For the reaction 2KClO₃ → 2KCl + 3O₂, the mole ratio of KClO₃ to O₂ is 2:3, meaning 2 moles of potassium chlorate produce 3 moles of oxygen.
Understanding limiting reactants helps you figure out which substance gets used up first and limits how much product you can make. The percent yield compares what you actually get in a reaction to what you theoretically should get.
Gas laws like Boyle's Law (pressure and volume are inversely related) and Gay-Lussac's Law (pressure and temperature are directly related) explain how gases behave under different conditions. These relationships are crucial for solving gas problems.
Real-World Connection: Percent yield calculations are used in pharmaceutical manufacturing to determine how efficient drug production processes are.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The ideal gas law is your ultimate tool for solving gas problems. This equation connects pressure, volume, temperature, and the amount of gas in one powerful formula that works for most real-world situations.
When working with gas mixtures, Dalton's Law states that total pressure equals the sum of all partial pressures. Partial pressure is the pressure each gas would exert if it were alone in the container.
Mole fraction helps you calculate partial pressures by showing what fraction of the total moles each gas contributes. Simply multiply the total pressure by the mole fraction to get the partial pressure of any gas in a mixture.
For gaseous reactions, you can use stoichiometry just like with other reactions. The volume ratios often match the mole ratios from the balanced equation when temperature and pressure stay constant.
Problem-Solving Tip: Always convert temperature to Kelvin and make sure your units match when using the ideal gas law - this prevents most calculation errors.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Graham's Law explains why some gases escape from containers faster than others. The rate of effusion (how fast gas particles escape through tiny holes) depends on the gas's molar mass - lighter gases move faster than heavier ones.
The mathematical relationship shows that effusion rate is inversely proportional to the square root of molar mass. This means hydrogen gas (very light) effuses much faster than ammonia gas (heavier).
You can use this principle to compare effusion rates of different gases or even identify unknown gases based on how quickly they escape. The graph shows this relationship clearly - as molar mass increases, the effusion rate decreases.
This concept connects to kinetic molecular theory, which explains that lighter gas particles move faster at the same temperature. Understanding this helps explain many real-world phenomena, like why helium balloons deflate faster than regular air-filled balloons.
Lab Application: Graham's Law is often tested through experiments comparing how fast different gases diffuse through porous materials or escape from containers.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
9
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user