Welcome to the Chemistry Problem Solver guide! This summary walks... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
32
•
Dec 21, 2025
•
Anne
@anne6
Welcome to the Chemistry Problem Solver guide! This summary walks... Show more








Ever wondered how to calculate the exact amount of product in a chemical reaction? Stoichiometry is your answer! Let's break down some examples:
When working with chemical equations like C₁₈H₁₀N₂O₄ + H₂O₂ → Products, you need to use mole ratios from the balanced equation. For melanin oxidation, if 20g of a 9% H₂O₂ solution is used, you'd calculate the mass of H₂O₂ first (1.8g), then convert to moles and apply the mole ratio.
For reactions like 2BF₃ + 3H₂ → 2B + 6HF, identifying the limiting reactant is crucial. Compare the theoretical yields from each reactant - the one producing less product is your limiting reactant. For example, 0.10 mol BF₃ would produce 0.10 mol B, while 0.25 mol H₂ would yield 0.17 mol B, making BF₃ the limiting reactant.
💡 Quick Tip: Always convert to moles first, then apply mole ratios from the balanced equation, and finally convert to the requested unit (mass, volume, etc.).
When calculating product yield for 3C₉H₈ + 5O₂ → 3C₉O₂ + 4H₂O, the same process applies. If you have 3.4g O₂, you'd convert to moles, apply the mole ratio , and then calculate the mass of water produced.

Chemistry calculations get easier with practice! Let's examine another example with the reaction 3Zn + 2MnO₃ → Mo₂O₃ + 3ZnO.
When given 5.0g Zn and 2.4g MnO₃, you need to determine which reactant will be used up first. Converting to moles:
Next, compare how much product each could theoretically make using the balanced equation's mole ratios. Since MnO₃ would produce less ZnO, it's your limiting reactant, while Zn is in excess.
For reactions involving Mn₃O₄ and Al, you can calculate:
💡 Remember: The limiting reactant is completely consumed in the reaction, while some excess reactant remains unused.
When working with large numbers like 5.33 × 10²⁵ atoms Al, convert to moles first (using Avogadro's number), then apply the appropriate mole ratio from the balanced equation.
Ready to tackle more complex chemical calculations? Let's look at calculations for the reaction 2C₅₇H₁₁₀O₆ + 163O₂ → 114CO₂ + 110H₂O.
For gas volume calculations at STP (Standard Temperature and Pressure), remember that 1 mole of any gas occupies 22.4L. If you have 0.64 mol of C₅₇H₁₁₀O₆, you can find the volume of O₂ needed: 0.64 mol × × = 1168L O₂
Similarly, you can calculate the volume of CO₂ produced: 0.64 mol × × = 817L CO₂
⚠️ Watch Out: When converting between moles and volume for gases, always check if the conditions are at STP. If not, you'll need to use the ideal gas law .
You can also calculate the mass of reactant needed to produce a specific amount of product. For example, to find how many grams of C₅₇H₁₁₀O₆ would produce 55.56 mol H₂O, use the mole ratio and molar mass conversion: 55.56 mol H₂O × × = 900g C₅₇H₁₁₀O₆.

Let's look at a complete limiting reactant problem for 3NH₄NO₃ + Na₃PO₄ → (NH₄)₃PO₄ + 3NaNO₃ with 30.0g NH₄NO₃ and 50.0g Na₃PO₄.
To identify the limiting reactant, calculate the theoretical yield of NaNO₃ from each reactant:
Since NH₄NO₃ produces less NaNO₃, it's the limiting reactant. To find the amount of Na₃PO₄ remaining:
💡 Pro Tip: When calculating remaining reactant, first determine how much was used in the reaction based on the limiting reactant, then subtract from the initial amount.
The physics portion involves vector calculations. When working with direction and magnitude, break each vector into x and y components using sine and cosine, then add all components separately to find the resultant vector.

Let's practice with 2Mg + O₂ → 2MgO. If we have 2.2g Mg and 4.5L O₂, which is limiting?
For Mg: 2.2g × × × = 2.03L MgO For O₂: 4.5L × × × = 9.00L MgO
Since Mg produces less MgO (2.03L vs 9.00L), it's the limiting reactant.
Now for 3CaCO₃ + 2FePO₄ → Ca₃(PO₄)₂ + Fe₂(CO₃)₃ with 100g CaCO₃ and 45g FePO₄:
For CaCO₃: 100g × × × = 103.39g Ca₃(PO₄)₂ For FePO₄: 45g × × × = 46.27g Ca₃(PO₄)₂
Since FePO₄ produces less Ca₃(PO₄)₂, it's limiting. To find remaining CaCO₃:
💡 Quick Method: Calculate how much CaCO₃ would react with the limiting reactant, then subtract from the initial amount to find what's left over.
Used CaCO₃: 45g FePO₄ × × × = 44.06g Remaining CaCO₃: 100g - 44.06g = 55.9g CaCO₃

For CuCl₂ + 2NaNO₃ → Cu(NO₃)₂ + 2NaCl with 15g CuCl₂ and 20g NaNO₃:
Calculate theoretical NaCl yield from both reactants:
Since CuCl₂ produces less NaCl, it's the limiting reactant.
To find unreacted NaNO₃:
For percent yield calculation: Percent Yield = × 100 = × 100 = 86.66%
💡 Gas Law Application: For Boyle's Law , when pressure increases, volume decreases proportionally, and vice versa.
Example: If V₁ = 2.5L at P₁ = 1.5atm, then at P₂ = 1atm: V₂ = (P₁V₁)/P₂ = (1.5atm × 2.5L)/1atm = 3.75L

Finding empirical and molecular formulas is a key chemistry skill! Here's the process:
Example 1: With K (39.8g), Mn (27.8g), and O (32.5g):
Example 2: With Na (32.4g), S (22.6g), and O (45.0g):
💡 Formula Tip: The empirical formula shows the simplest whole-number ratio of atoms, while the molecular formula shows the actual number of atoms in the molecule.
For molecular formulas, determine the empirical formula first, calculate its mass, then find how many empirical formula units make up one molecule:
Let's solve a limiting reactant problem for the combustion reaction 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O:
With 16.4L C₂H₆ and 0.980 mol O₂, determine the limiting reactant by calculating CO₂ produced:
Since O₂ produces less CO₂, it's the limiting reactant.
To find remaining C₂H₆:
💡 Gas Law Applications: Know when to use which gas law!
- Boyle's Law : Relates pressure and volume at constant temperature
- Charles's Law : Relates volume and temperature at constant pressure
- Combined Gas Law:
Example: At 740 torr and 250mL, what volume would gas occupy at 600 torr? P₁V₁ = P₂V₂ (0.97atm)(0.25L) = (0.79atm)(V₂) V₂ = 0.23L or 230mL

Charles's Law relates volume and temperature at constant pressure. Let's see it in action:
For a gas at 250mL (0.25L) and 25°C (298.15K), what would the volume be at 95°C (368.15K)? V₁/T₁ = V₂/T₂ 0.25L/298.15K = V₂/368.15K V₂ = (0.25L × 368.15K)/298.15K = 0.31L or 308.7mL
The Combined Gas Law relates pressure, volume, and temperature: For a gas at 256mL (0.26L), 720 torr (0.95atm), and 25°C (298.15K), what's the pressure when the volume is 245mL (0.24L) at 50°C (323.15K)? (P₁V₁)/T₁ = (P₂V₂)/T₂ P₂ = (P₁V₁T₂)/(T₁V₂) = (0.95atm × 0.26L × 323.15K)/(298.15K × 0.24L) = 1.12atm or 848 torr
💡 Temperature Conversion: Always convert temperature to Kelvin for gas law calculations by adding 273.15 to the Celsius temperature.
Using Boyle's Law again: If a 3.50L gas at 125kPa (1.23atm) is compressed to 2.00L, the new pressure is: P₂ = (P₁V₁)/V₂ = (1.23atm × 3.50L)/2.00L = 2.15atm or 219kPa
Finally, the Ideal Gas Law can find the number of moles: For 3.9L of gas at 1.00atm and 40°C (313.15K): n = PV/(RT) = (1.00atm × 3.9L)/ = 0.15117 mol
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Anne
@anne6
Welcome to the Chemistry Problem Solver guide! This summary walks you through key chemistry calculations including stoichiometry, limiting reactants, percent yield, and gas laws. You'll find step-by-step solutions that show exactly how to tackle these common chemistry problems using conversion... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ever wondered how to calculate the exact amount of product in a chemical reaction? Stoichiometry is your answer! Let's break down some examples:
When working with chemical equations like C₁₈H₁₀N₂O₄ + H₂O₂ → Products, you need to use mole ratios from the balanced equation. For melanin oxidation, if 20g of a 9% H₂O₂ solution is used, you'd calculate the mass of H₂O₂ first (1.8g), then convert to moles and apply the mole ratio.
For reactions like 2BF₃ + 3H₂ → 2B + 6HF, identifying the limiting reactant is crucial. Compare the theoretical yields from each reactant - the one producing less product is your limiting reactant. For example, 0.10 mol BF₃ would produce 0.10 mol B, while 0.25 mol H₂ would yield 0.17 mol B, making BF₃ the limiting reactant.
💡 Quick Tip: Always convert to moles first, then apply mole ratios from the balanced equation, and finally convert to the requested unit (mass, volume, etc.).
When calculating product yield for 3C₉H₈ + 5O₂ → 3C₉O₂ + 4H₂O, the same process applies. If you have 3.4g O₂, you'd convert to moles, apply the mole ratio , and then calculate the mass of water produced.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemistry calculations get easier with practice! Let's examine another example with the reaction 3Zn + 2MnO₃ → Mo₂O₃ + 3ZnO.
When given 5.0g Zn and 2.4g MnO₃, you need to determine which reactant will be used up first. Converting to moles:
Next, compare how much product each could theoretically make using the balanced equation's mole ratios. Since MnO₃ would produce less ZnO, it's your limiting reactant, while Zn is in excess.
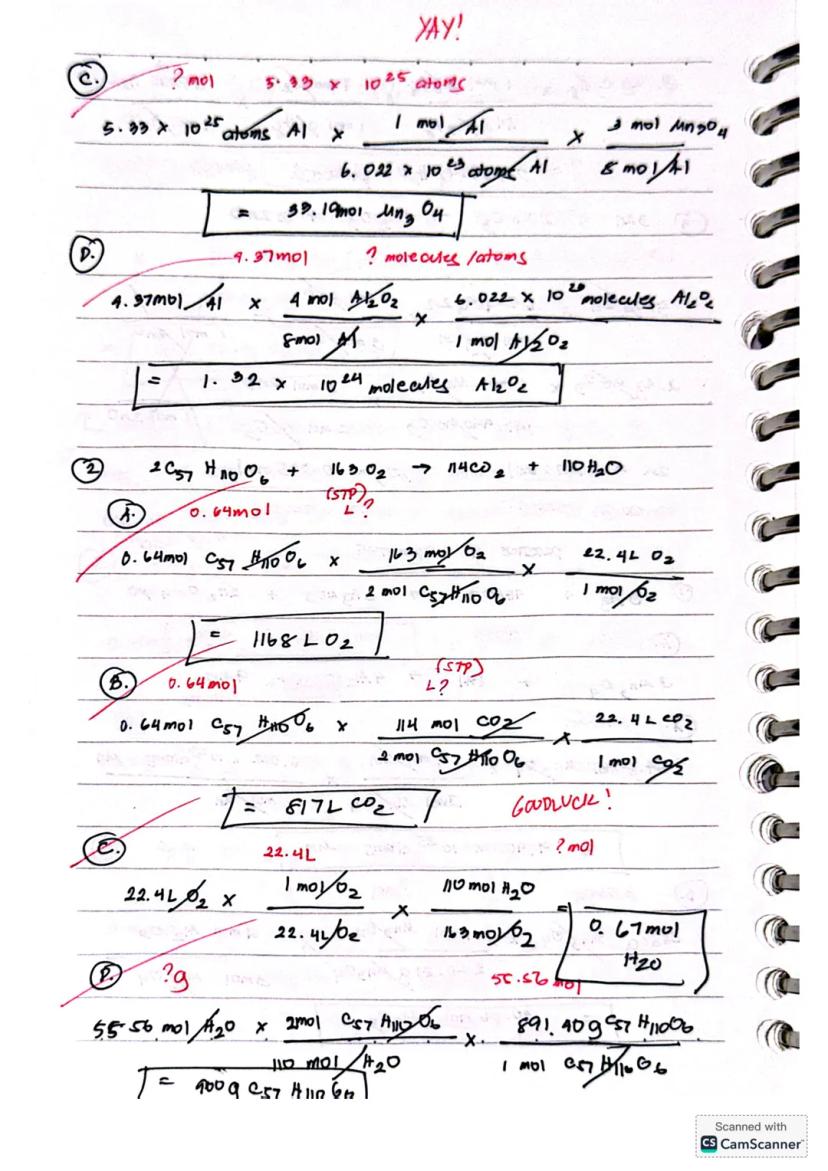
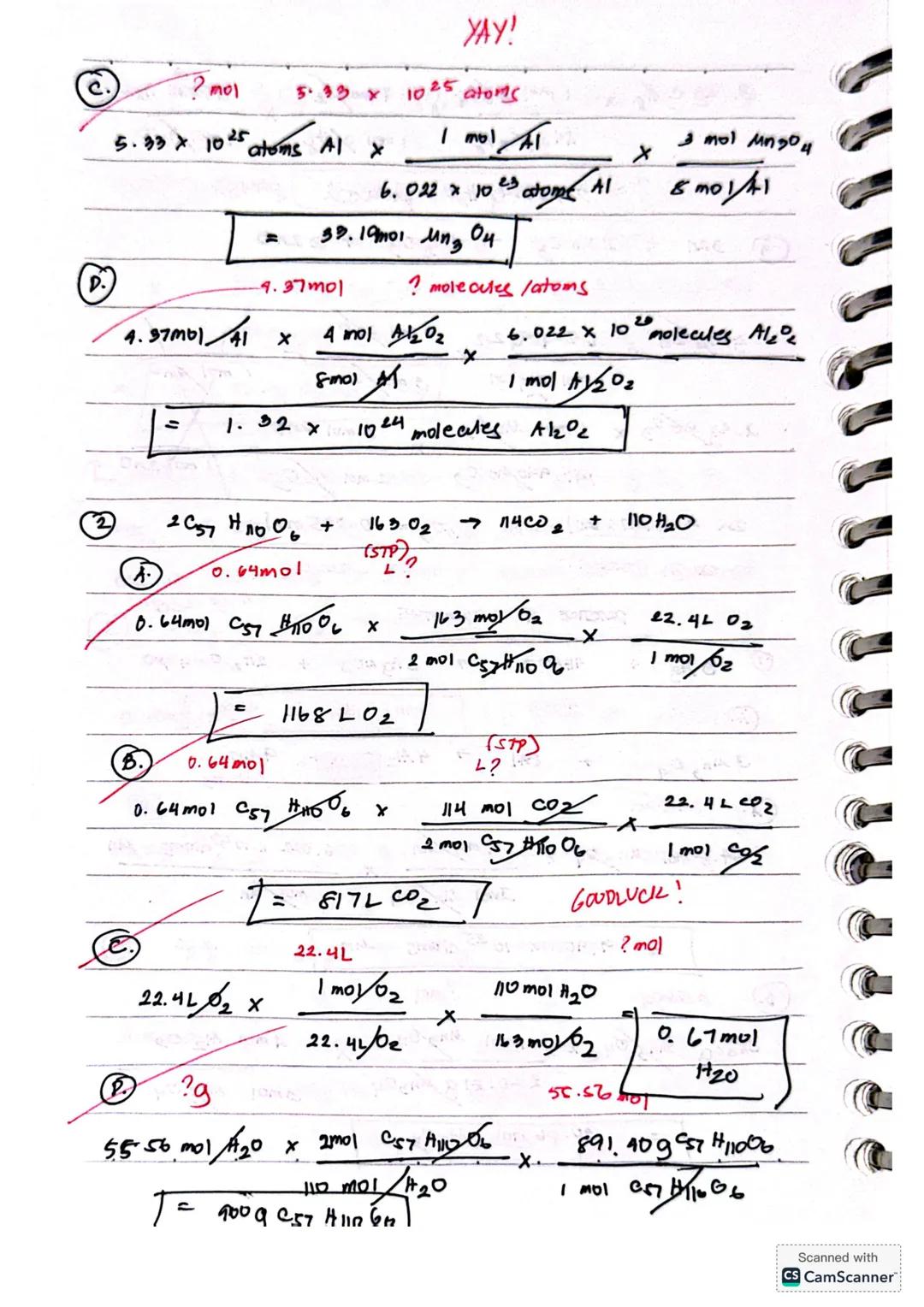
For reactions involving Mn₃O₄ and Al, you can calculate:
💡 Remember: The limiting reactant is completely consumed in the reaction, while some excess reactant remains unused.
When working with large numbers like 5.33 × 10²⁵ atoms Al, convert to moles first (using Avogadro's number), then apply the appropriate mole ratio from the balanced equation.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Ready to tackle more complex chemical calculations? Let's look at calculations for the reaction 2C₅₇H₁₁₀O₆ + 163O₂ → 114CO₂ + 110H₂O.
For gas volume calculations at STP (Standard Temperature and Pressure), remember that 1 mole of any gas occupies 22.4L. If you have 0.64 mol of C₅₇H₁₁₀O₆, you can find the volume of O₂ needed: 0.64 mol × × = 1168L O₂
Similarly, you can calculate the volume of CO₂ produced: 0.64 mol × × = 817L CO₂
⚠️ Watch Out: When converting between moles and volume for gases, always check if the conditions are at STP. If not, you'll need to use the ideal gas law .
You can also calculate the mass of reactant needed to produce a specific amount of product. For example, to find how many grams of C₅₇H₁₁₀O₆ would produce 55.56 mol H₂O, use the mole ratio and molar mass conversion: 55.56 mol H₂O × × = 900g C₅₇H₁₁₀O₆.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Let's look at a complete limiting reactant problem for 3NH₄NO₃ + Na₃PO₄ → (NH₄)₃PO₄ + 3NaNO₃ with 30.0g NH₄NO₃ and 50.0g Na₃PO₄.
To identify the limiting reactant, calculate the theoretical yield of NaNO₃ from each reactant:
Since NH₄NO₃ produces less NaNO₃, it's the limiting reactant. To find the amount of Na₃PO₄ remaining:
💡 Pro Tip: When calculating remaining reactant, first determine how much was used in the reaction based on the limiting reactant, then subtract from the initial amount.
The physics portion involves vector calculations. When working with direction and magnitude, break each vector into x and y components using sine and cosine, then add all components separately to find the resultant vector.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Let's practice with 2Mg + O₂ → 2MgO. If we have 2.2g Mg and 4.5L O₂, which is limiting?
For Mg: 2.2g × × × = 2.03L MgO For O₂: 4.5L × × × = 9.00L MgO
Since Mg produces less MgO (2.03L vs 9.00L), it's the limiting reactant.
Now for 3CaCO₃ + 2FePO₄ → Ca₃(PO₄)₂ + Fe₂(CO₃)₃ with 100g CaCO₃ and 45g FePO₄:
For CaCO₃: 100g × × × = 103.39g Ca₃(PO₄)₂ For FePO₄: 45g × × × = 46.27g Ca₃(PO₄)₂
Since FePO₄ produces less Ca₃(PO₄)₂, it's limiting. To find remaining CaCO₃:
💡 Quick Method: Calculate how much CaCO₃ would react with the limiting reactant, then subtract from the initial amount to find what's left over.
Used CaCO₃: 45g FePO₄ × × × = 44.06g Remaining CaCO₃: 100g - 44.06g = 55.9g CaCO₃

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
For CuCl₂ + 2NaNO₃ → Cu(NO₃)₂ + 2NaCl with 15g CuCl₂ and 20g NaNO₃:
Calculate theoretical NaCl yield from both reactants:
Since CuCl₂ produces less NaCl, it's the limiting reactant.
To find unreacted NaNO₃:
For percent yield calculation: Percent Yield = × 100 = × 100 = 86.66%
💡 Gas Law Application: For Boyle's Law , when pressure increases, volume decreases proportionally, and vice versa.
Example: If V₁ = 2.5L at P₁ = 1.5atm, then at P₂ = 1atm: V₂ = (P₁V₁)/P₂ = (1.5atm × 2.5L)/1atm = 3.75L

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Finding empirical and molecular formulas is a key chemistry skill! Here's the process:
Example 1: With K (39.8g), Mn (27.8g), and O (32.5g):
Example 2: With Na (32.4g), S (22.6g), and O (45.0g):
💡 Formula Tip: The empirical formula shows the simplest whole-number ratio of atoms, while the molecular formula shows the actual number of atoms in the molecule.
For molecular formulas, determine the empirical formula first, calculate its mass, then find how many empirical formula units make up one molecule:

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
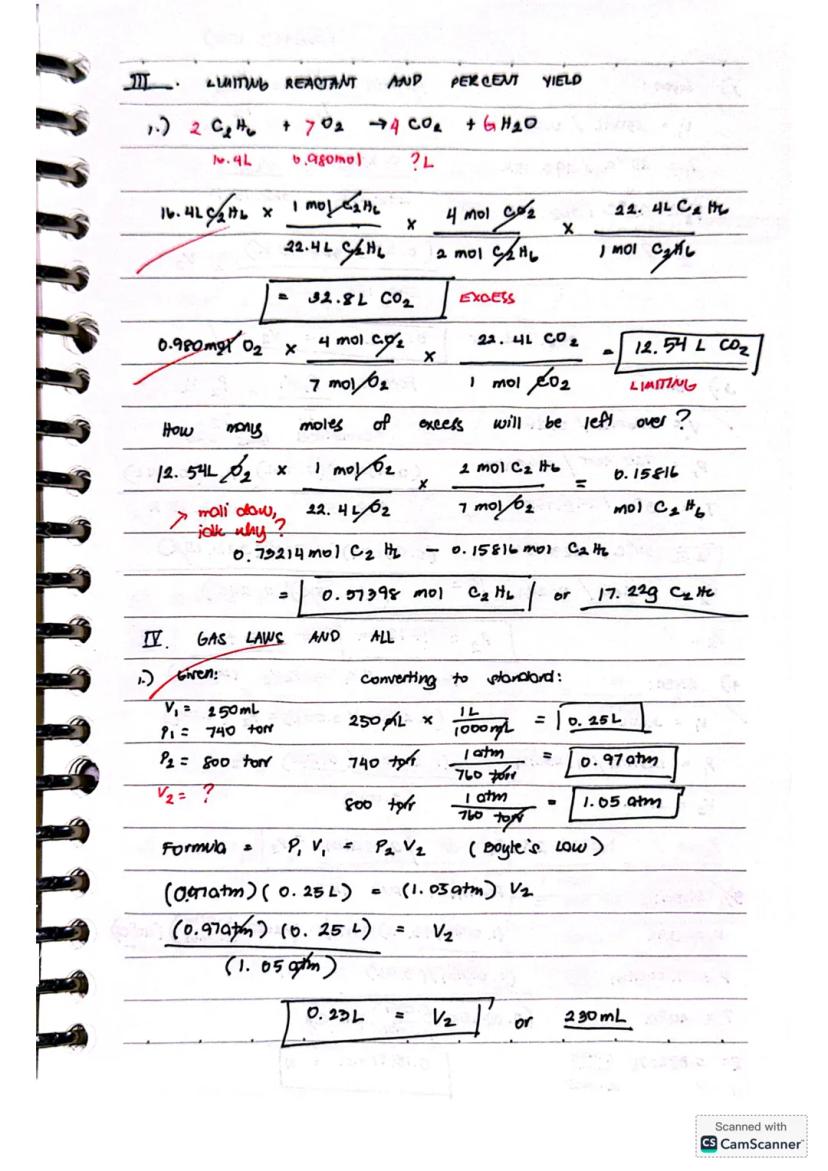
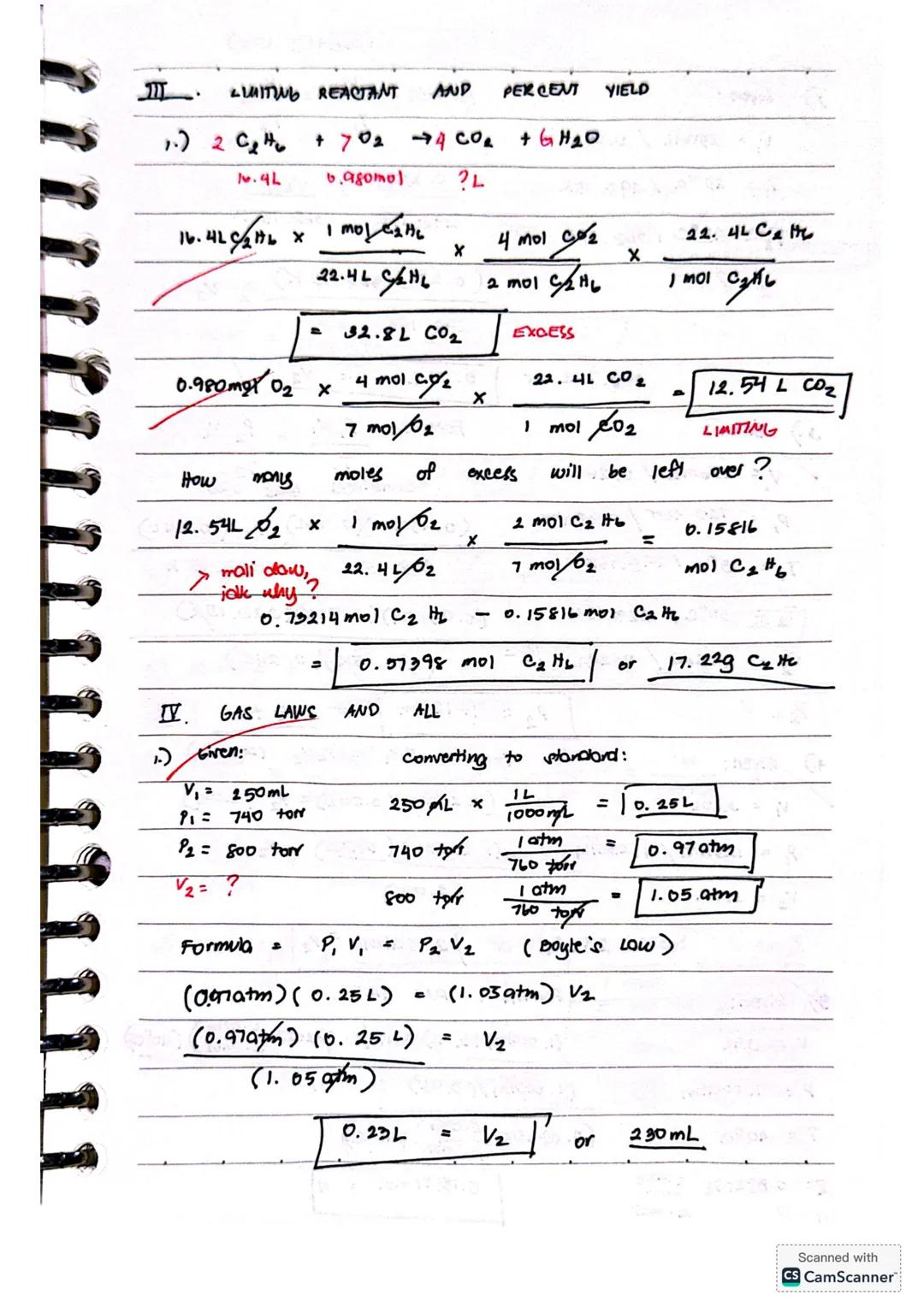
Let's solve a limiting reactant problem for the combustion reaction 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O:
With 16.4L C₂H₆ and 0.980 mol O₂, determine the limiting reactant by calculating CO₂ produced:
Since O₂ produces less CO₂, it's the limiting reactant.
To find remaining C₂H₆:
💡 Gas Law Applications: Know when to use which gas law!
- Boyle's Law : Relates pressure and volume at constant temperature
- Charles's Law : Relates volume and temperature at constant pressure
- Combined Gas Law:
Example: At 740 torr and 250mL, what volume would gas occupy at 600 torr? P₁V₁ = P₂V₂ (0.97atm)(0.25L) = (0.79atm)(V₂) V₂ = 0.23L or 230mL

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Charles's Law relates volume and temperature at constant pressure. Let's see it in action:
For a gas at 250mL (0.25L) and 25°C (298.15K), what would the volume be at 95°C (368.15K)? V₁/T₁ = V₂/T₂ 0.25L/298.15K = V₂/368.15K V₂ = (0.25L × 368.15K)/298.15K = 0.31L or 308.7mL
The Combined Gas Law relates pressure, volume, and temperature: For a gas at 256mL (0.26L), 720 torr (0.95atm), and 25°C (298.15K), what's the pressure when the volume is 245mL (0.24L) at 50°C (323.15K)? (P₁V₁)/T₁ = (P₂V₂)/T₂ P₂ = (P₁V₁T₂)/(T₁V₂) = (0.95atm × 0.26L × 323.15K)/(298.15K × 0.24L) = 1.12atm or 848 torr
💡 Temperature Conversion: Always convert temperature to Kelvin for gas law calculations by adding 273.15 to the Celsius temperature.
Using Boyle's Law again: If a 3.50L gas at 125kPa (1.23atm) is compressed to 2.00L, the new pressure is: P₂ = (P₁V₁)/V₂ = (1.23atm × 3.50L)/2.00L = 2.15atm or 219kPa
Finally, the Ideal Gas Law can find the number of moles: For 3.9L of gas at 1.00atm and 40°C (313.15K): n = PV/(RT) = (1.00atm × 3.9L)/ = 0.15117 mol
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
2
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user