Electron Configuration and Periodic Trends
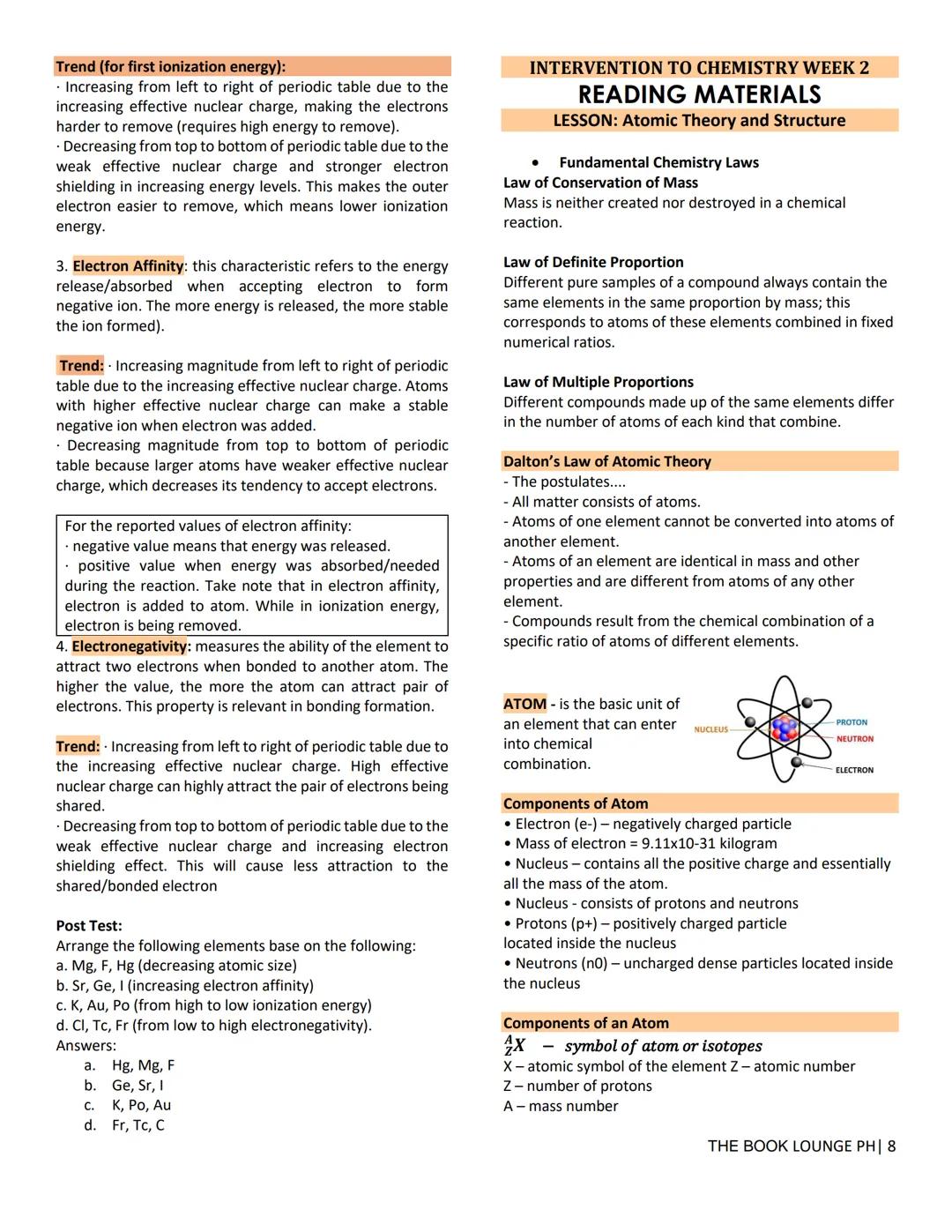
Electrons aren't randomly scattered around the nucleus - they occupy specific energy levels and shapes called orbitals. Understanding this electron arrangement explains why elements behave so differently.
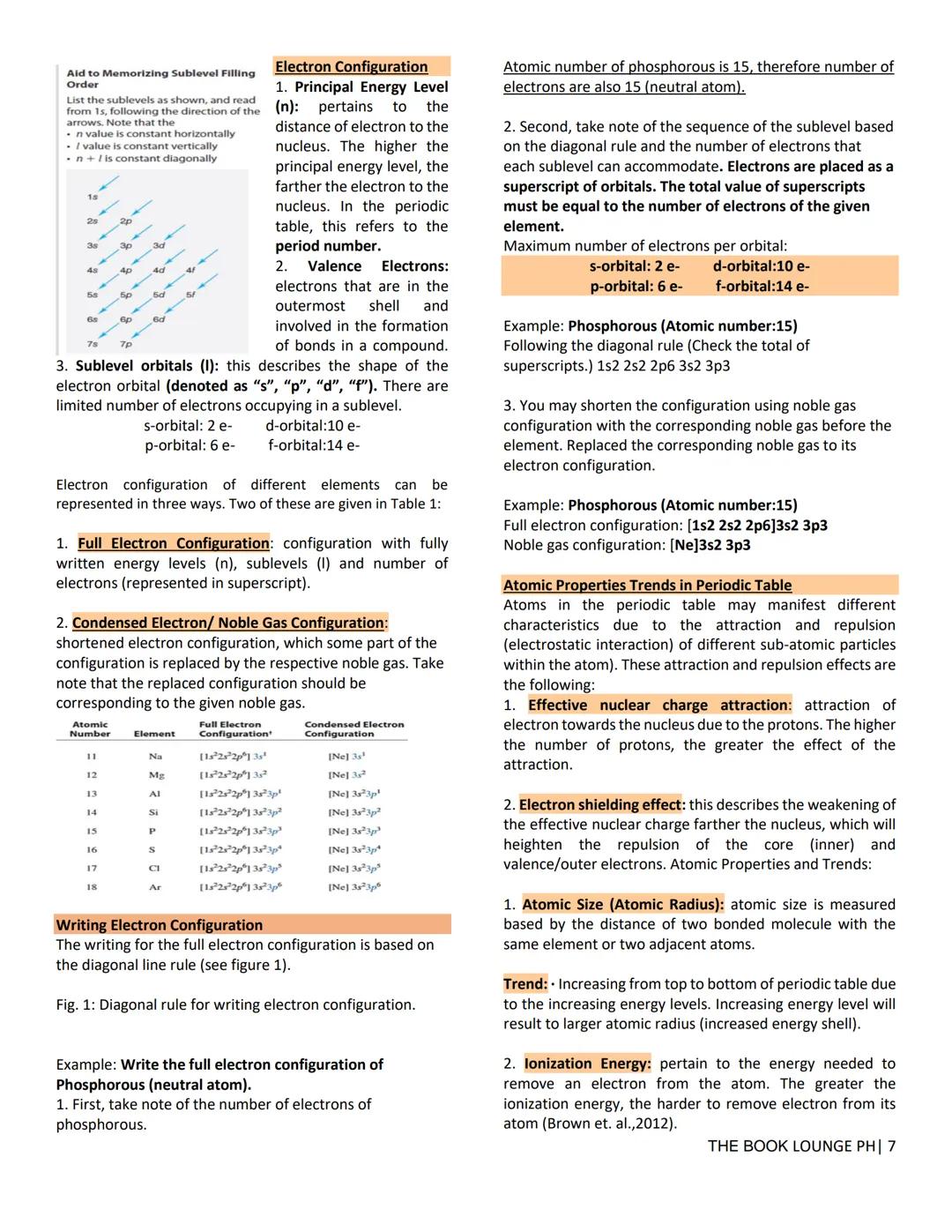
Energy levels n=1,2,3... determine distance from the nucleus, with higher levels farther out. Each level contains sublevels (s, p, d, f) with different shapes and capacities: s holds 2 electrons, p holds 6, d holds 10, and f holds 14.
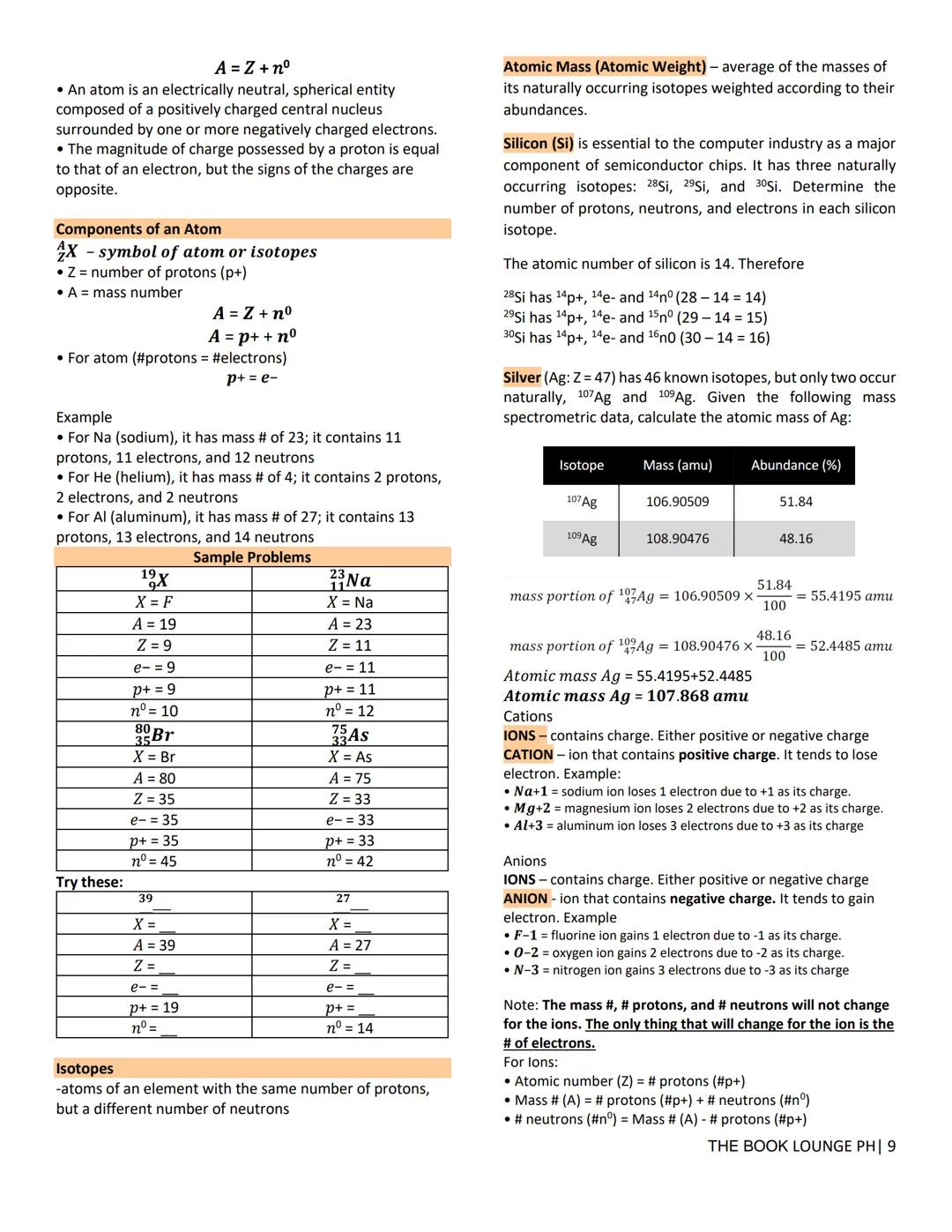
Writing electron configurations follows the diagonal rule, filling lowest energy orbitals first. For example, phosphorus (15 electrons) is 1s² 2s² 2p⁶ 3s² 3p³. Noble gas notation shortens this to Ne 3s² 3p³ by replacing inner electrons with the previous noble gas.
Periodic trends result from electron-nucleus interactions. Atomic size increases down groups (more energy levels) and decreases across periods (stronger nuclear pull). Ionization energy (energy to remove an electron) increases across periods and decreases down groups. Electronegativity (attraction for bonding electrons) follows the same pattern as ionization energy.
Memory Trick: Think of nuclear charge like a magnet - the stronger it gets (more protons), the tighter it holds electrons, making atoms smaller and harder to ionize!