Chemical reactions don't all happen at the same speed -... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
288
•
Dec 29, 2025
•
Chemical reactions don't all happen at the same speed -... Show more
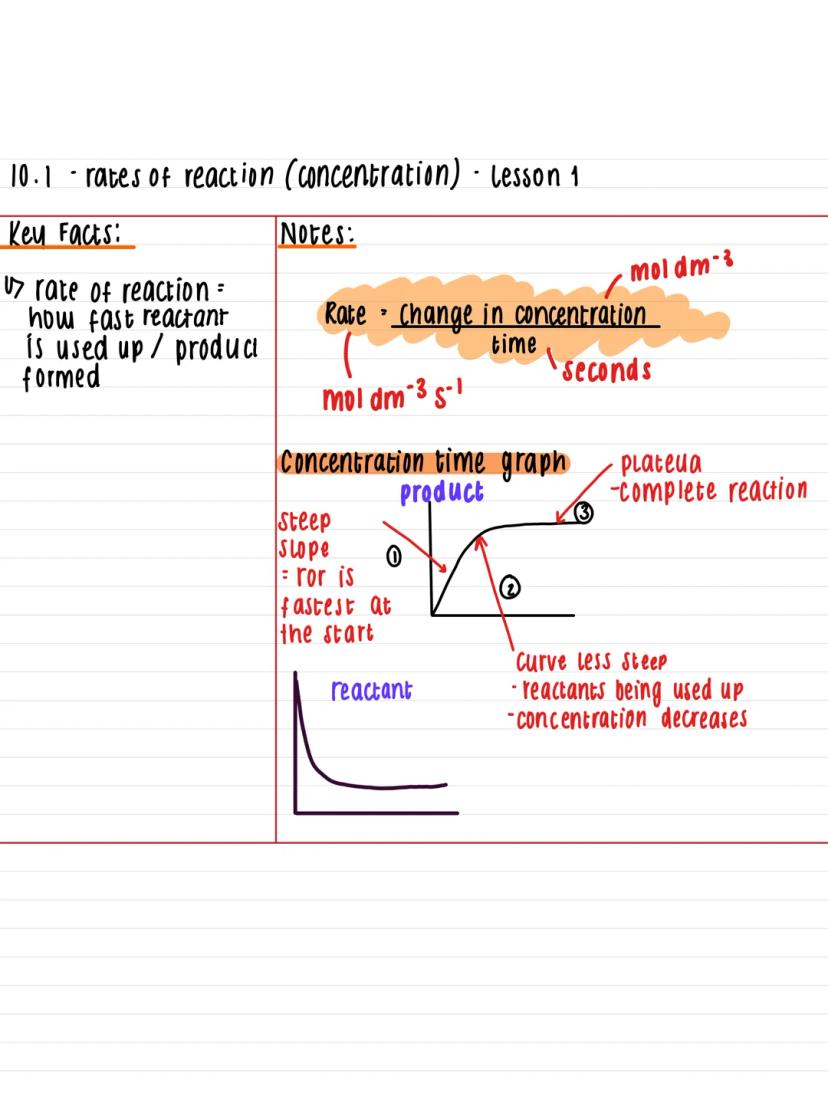

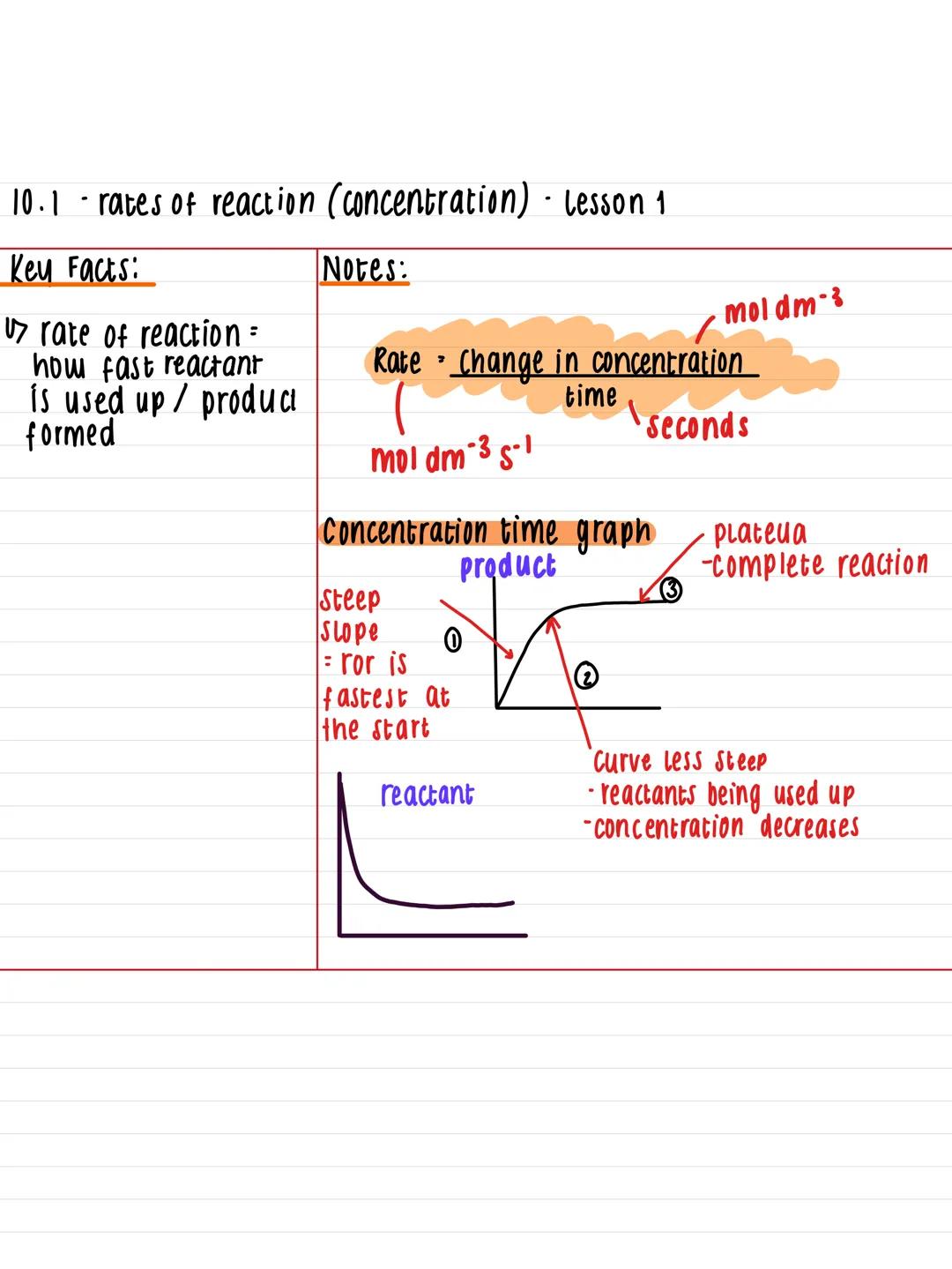
Think of rate of reaction as the speedometer for chemical processes - it measures how quickly reactants disappear or products form. You'll calculate this using the formula: rate = change in concentration ÷ time, with units of mol dm⁻³ s⁻¹.
When you plot concentration against time, you'll get a distinctive curve that tells an important story. The graph starts steep because reactions are fastest at the beginning when there's loads of reactant available. As time goes on, the curve becomes less steep because reactants get used up.
Eventually, the line flattens into a plateau, showing the reaction has finished. This pattern appears in virtually every concentration-time graph you'll encounter, so recognising it is dead useful for your exams.
Quick Tip: The steeper the slope at any point, the faster the reaction is happening at that moment!

Four main factors control how fast reactions happen: concentration, temperature, catalyst presence, and surface area. Each one works by affecting how particles bump into each other during the reaction.
Collision theory explains why these factors matter so much. For a reaction to occur, particles must collide with the correct orientation and have energy greater than the activation energy. It's like trying to unlock a door - you need the right key (orientation) and enough force to turn it (energy).
Concentration has a massive impact on reaction rates. Higher concentration means more particles crammed into the same space, leading to more frequent successful collisions and a faster reaction. Lower concentration gives you fewer particles, fewer collisions, and a slower rate.
Remember: More particles = more crashes = faster reactions!
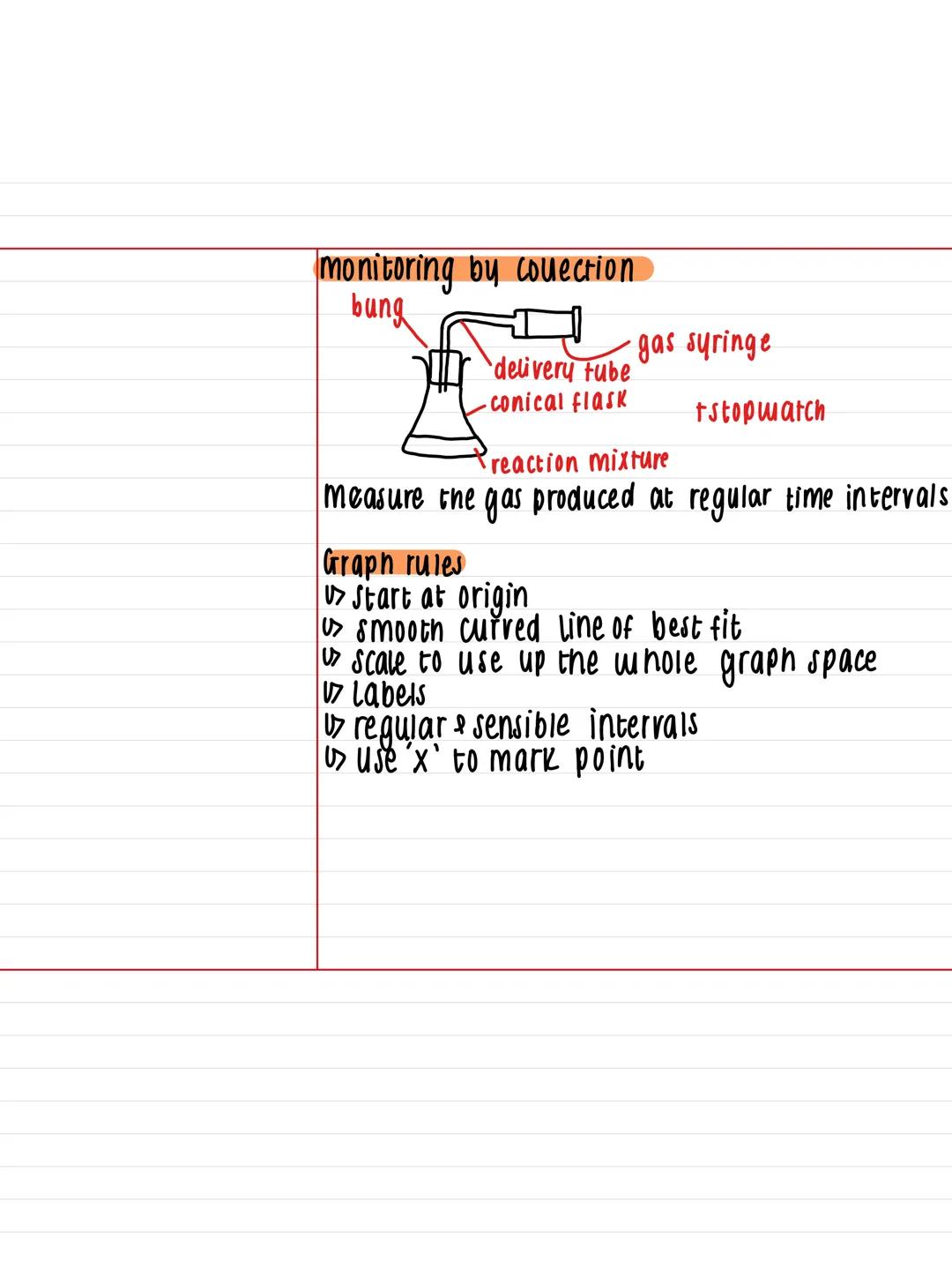
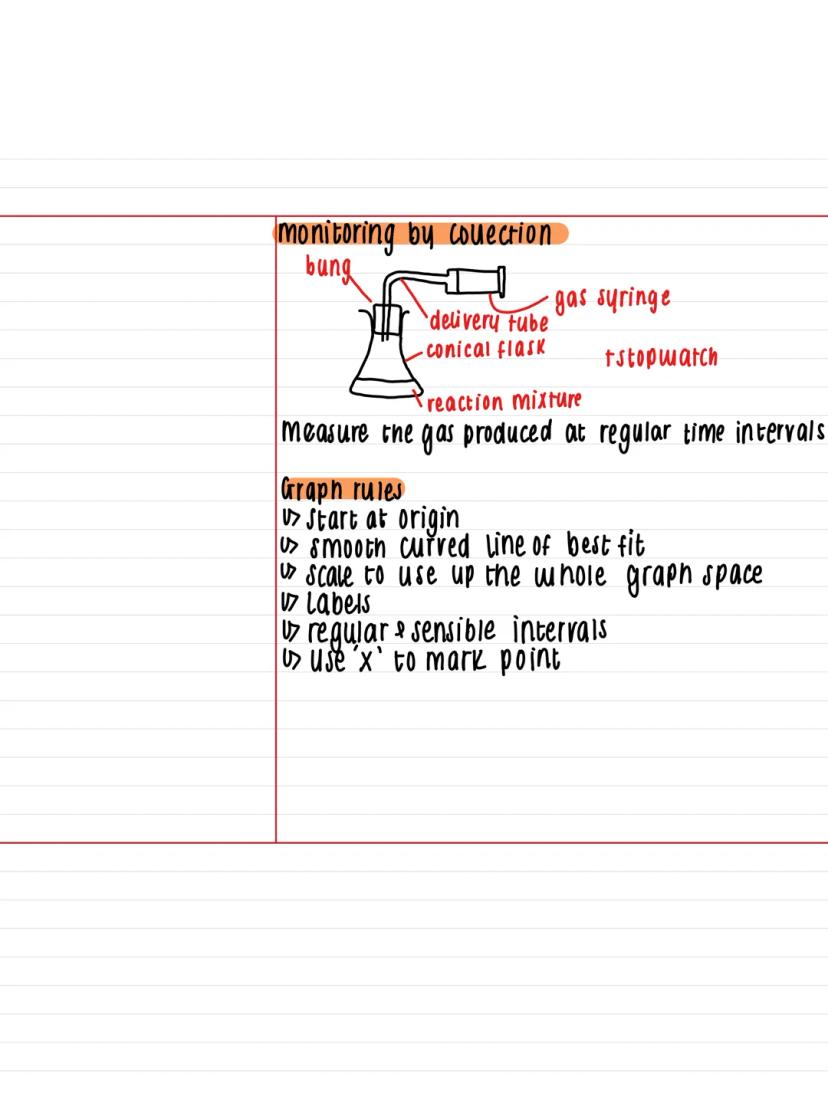
One brilliant way to monitor reaction rates is by collecting gas using simple lab equipment. You'll need a conical flask for your reaction mixture, connected to a gas syringe via a delivery tube, with a bung sealing everything up.
The method is straightforward: measure the volume of gas produced at regular time intervals using your stopwatch. This gives you real-time data about how fast your reaction is progressing.
When plotting your results, follow these essential graph rules: start at the origin, draw smooth curved lines of best fit, and use sensible scale intervals that fill the whole graph space. Mark each data point with an 'x' and ensure your axes are properly labelled.
Pro Tip: Using the full graph space makes it much easier to read accurate values and spot trends in your data!
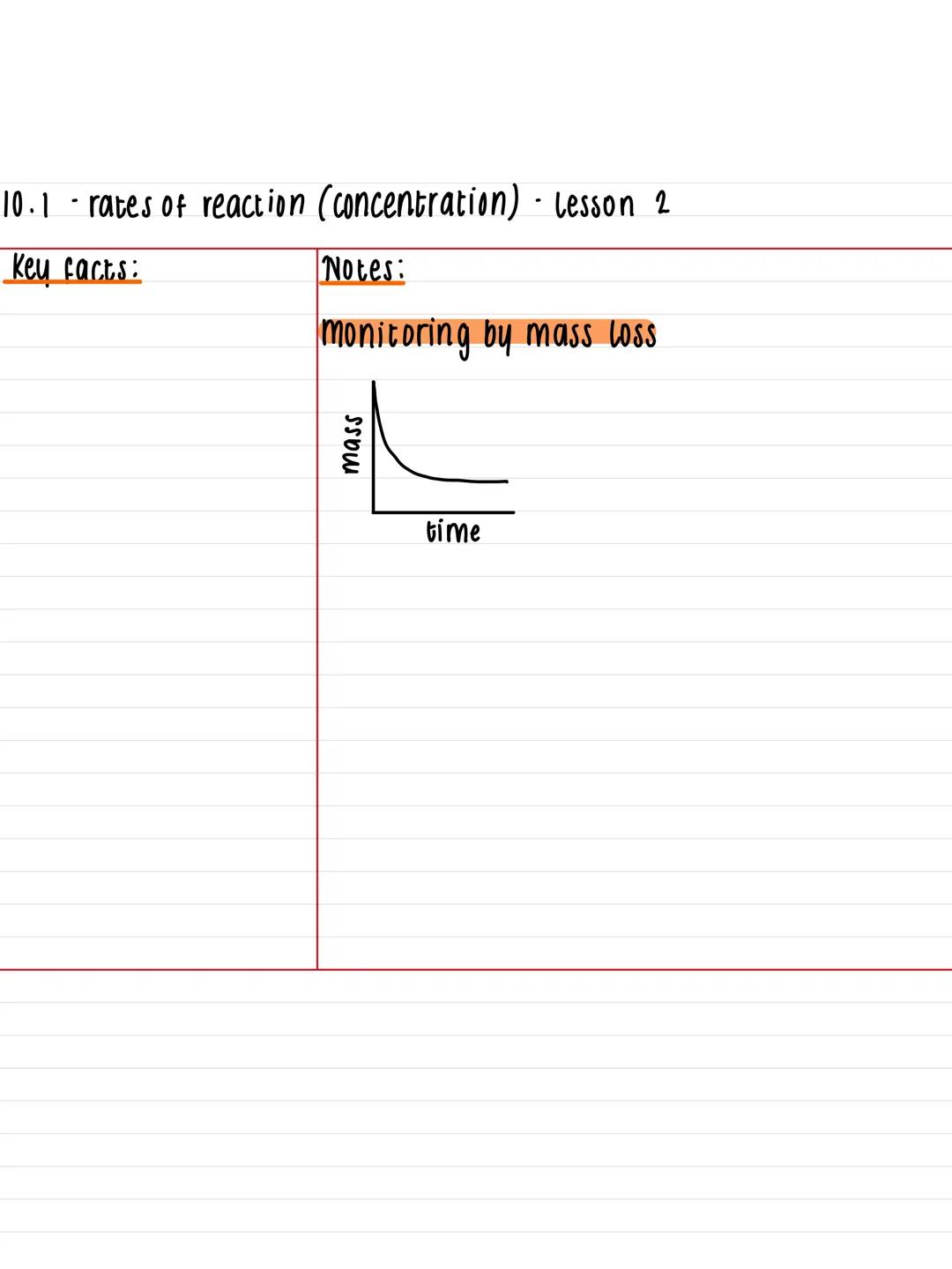
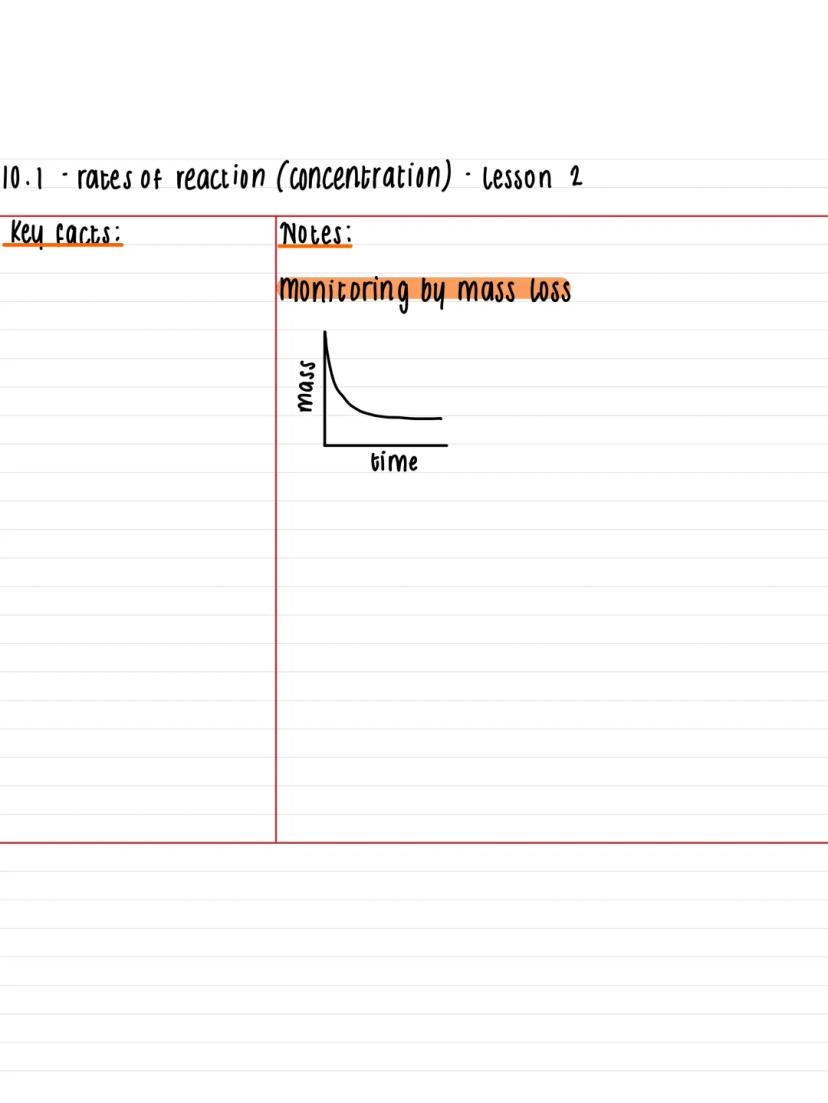
Mass loss provides another excellent method for tracking reaction rates, especially when gases escape from your reaction mixture. As the reaction proceeds and gas bubbles away, the total mass of your setup decreases over time.
This technique works particularly well for reactions producing carbon dioxide, hydrogen, or other gases that can escape. You'll plot mass against time to create graphs that show the same characteristic curve pattern as concentration-time graphs.
The beauty of this method is its simplicity - just pop your reaction vessel on a balance and record the mass at regular intervals. The faster the mass drops, the quicker your reaction is happening.
Key Point: Mass loss and gas collection often give you the same information, so choose the method that works best for your specific reaction!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Chemical reactions don't all happen at the same speed - some are lightning fast whilst others take ages to complete. Understanding rates of reactionis crucial for your chemistry exams and helps explain everything from why food spoils to how... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of rate of reaction as the speedometer for chemical processes - it measures how quickly reactants disappear or products form. You'll calculate this using the formula: rate = change in concentration ÷ time, with units of mol dm⁻³ s⁻¹.
When you plot concentration against time, you'll get a distinctive curve that tells an important story. The graph starts steep because reactions are fastest at the beginning when there's loads of reactant available. As time goes on, the curve becomes less steep because reactants get used up.
Eventually, the line flattens into a plateau, showing the reaction has finished. This pattern appears in virtually every concentration-time graph you'll encounter, so recognising it is dead useful for your exams.
Quick Tip: The steeper the slope at any point, the faster the reaction is happening at that moment!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Four main factors control how fast reactions happen: concentration, temperature, catalyst presence, and surface area. Each one works by affecting how particles bump into each other during the reaction.
Collision theory explains why these factors matter so much. For a reaction to occur, particles must collide with the correct orientation and have energy greater than the activation energy. It's like trying to unlock a door - you need the right key (orientation) and enough force to turn it (energy).
Concentration has a massive impact on reaction rates. Higher concentration means more particles crammed into the same space, leading to more frequent successful collisions and a faster reaction. Lower concentration gives you fewer particles, fewer collisions, and a slower rate.
Remember: More particles = more crashes = faster reactions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
One brilliant way to monitor reaction rates is by collecting gas using simple lab equipment. You'll need a conical flask for your reaction mixture, connected to a gas syringe via a delivery tube, with a bung sealing everything up.
The method is straightforward: measure the volume of gas produced at regular time intervals using your stopwatch. This gives you real-time data about how fast your reaction is progressing.
When plotting your results, follow these essential graph rules: start at the origin, draw smooth curved lines of best fit, and use sensible scale intervals that fill the whole graph space. Mark each data point with an 'x' and ensure your axes are properly labelled.
Pro Tip: Using the full graph space makes it much easier to read accurate values and spot trends in your data!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Mass loss provides another excellent method for tracking reaction rates, especially when gases escape from your reaction mixture. As the reaction proceeds and gas bubbles away, the total mass of your setup decreases over time.
This technique works particularly well for reactions producing carbon dioxide, hydrogen, or other gases that can escape. You'll plot mass against time to create graphs that show the same characteristic curve pattern as concentration-time graphs.
The beauty of this method is its simplicity - just pop your reaction vessel on a balance and record the mass at regular intervals. The faster the mass drops, the quicker your reaction is happening.
Key Point: Mass loss and gas collection often give you the same information, so choose the method that works best for your specific reaction!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
4
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
You will be able to learn how do we measure in science.
Learn about Ernest Rutherford's groundbreaking experiment that confirmed the presence of positive and negative charges within atoms and led to the discovery of the atomic nucleus.
Calculate percent error and density
Explore key concepts of reaction rates, including collision theory, factors affecting reaction speed, and the role of catalysts. This summary covers essential exam questions and explanations related to reaction kinetics, temperature effects, and chemical equilibrium. Ideal for students preparing for chemistry assessments.
Learn about the reactivity and properties of metals, alkali metals, alkaline earth metals, and transition metals in the periodic table.
An introduction to chemistry including the different branches of chemistry.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user