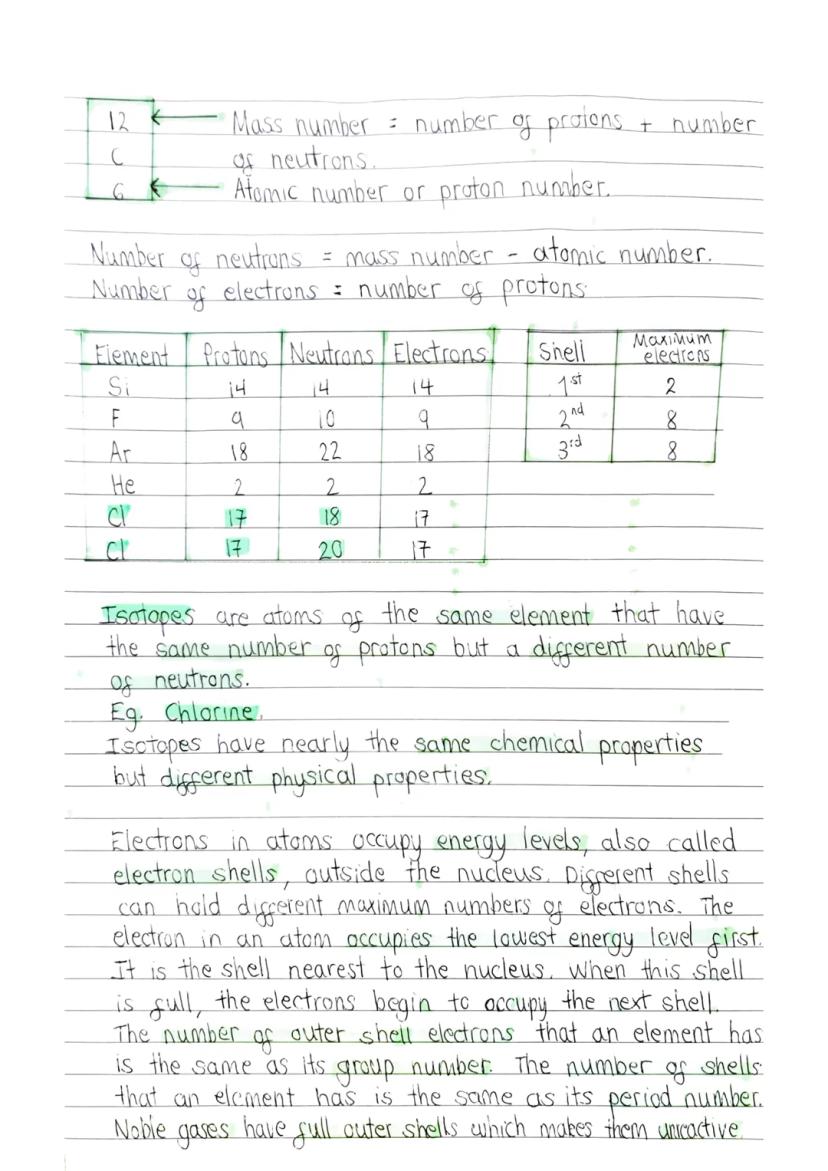
Chemical Bonding
Atoms bond together because they want full outer shells like the noble gases. There are two main types: ionic bonding betweenmetalsandnon−metals and covalent bonding betweennon−metalsonly.
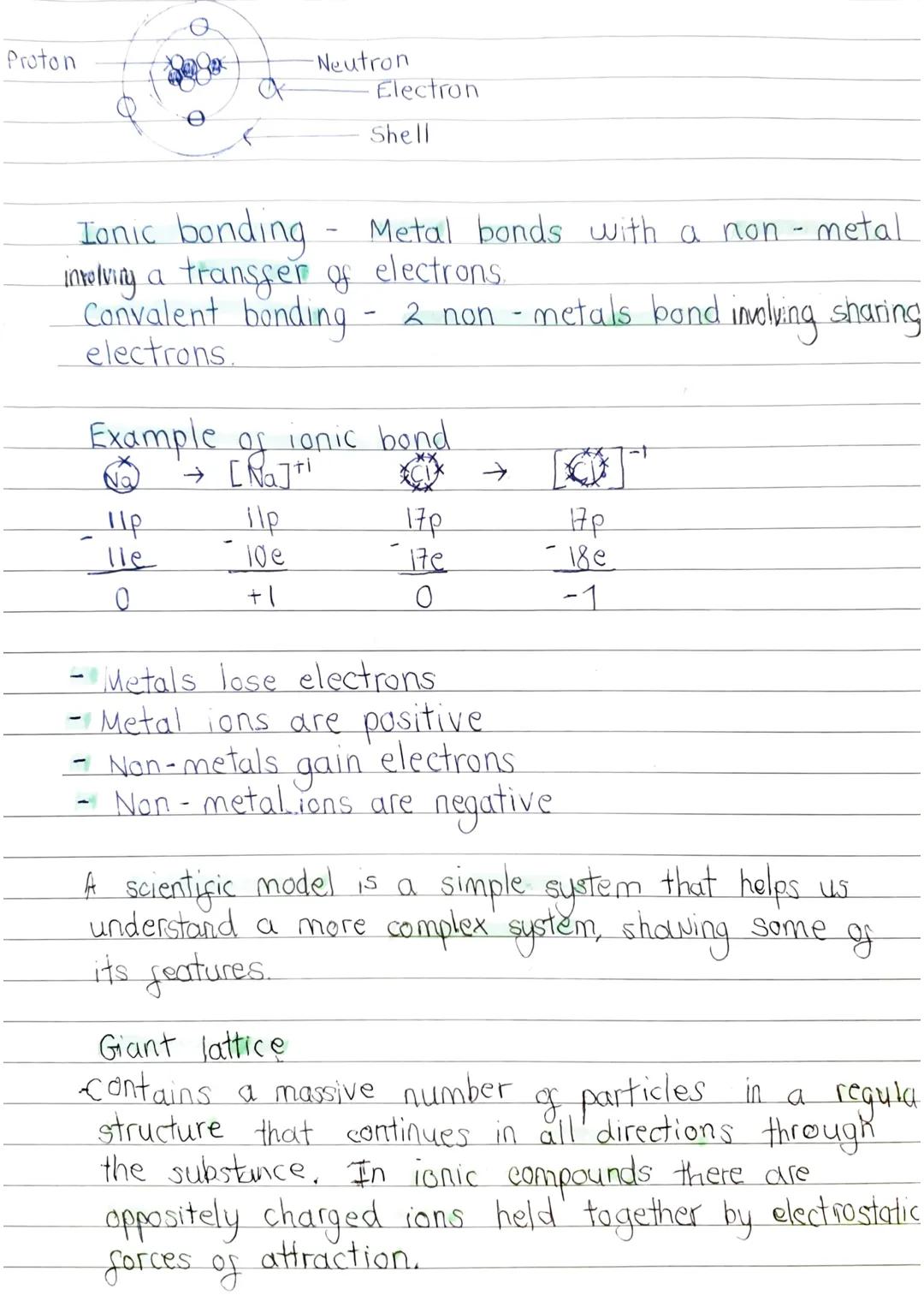
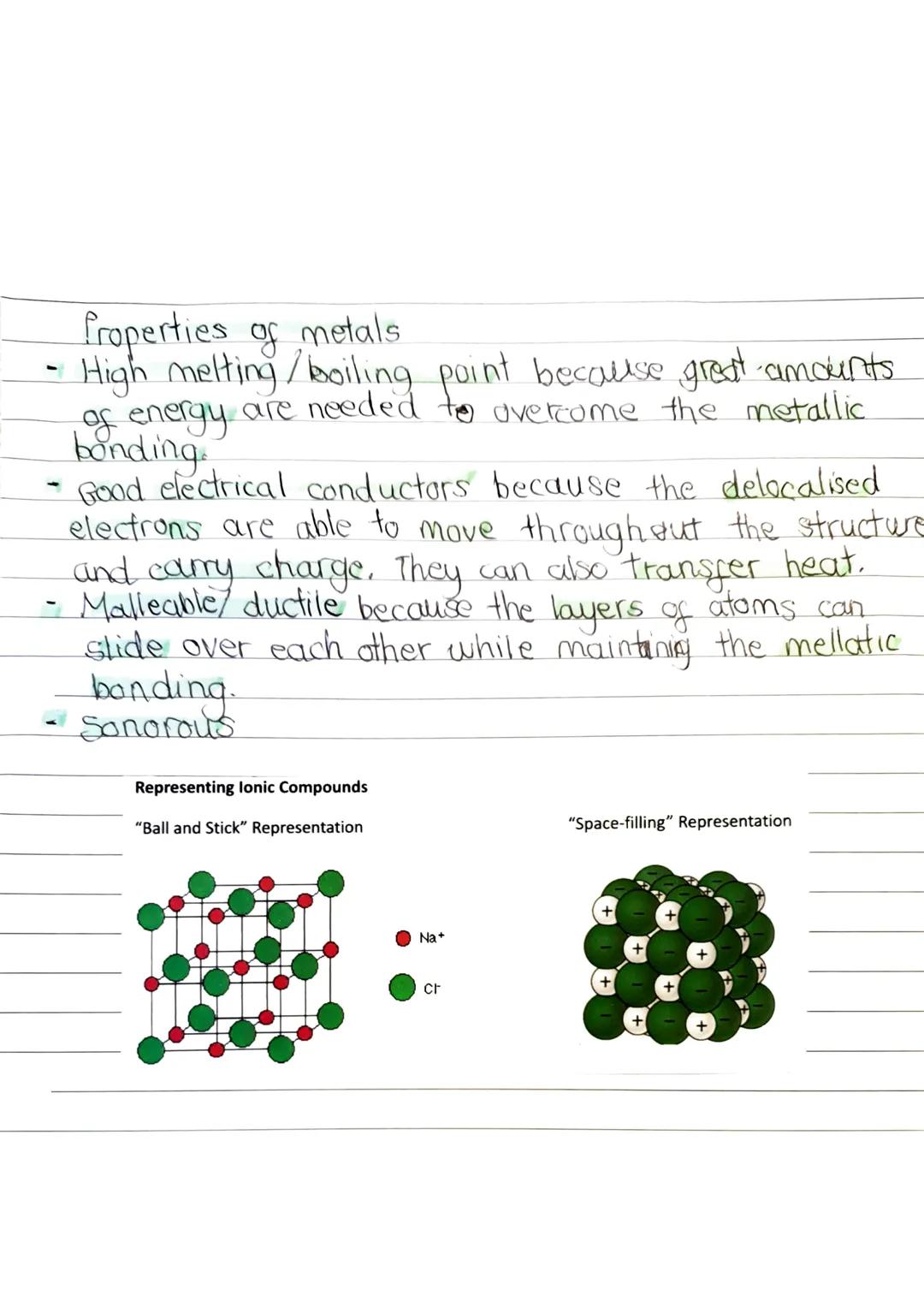
In ionic bonding, electrons transfer completely from metal to non-metal atoms. Metals lose electrons and become positive ions, whilst non-metals gain electrons and become negative ions. Think of sodium chloride: sodium gives up one electron to become Na⁺, and chlorine accepts it to become Cl⁻.
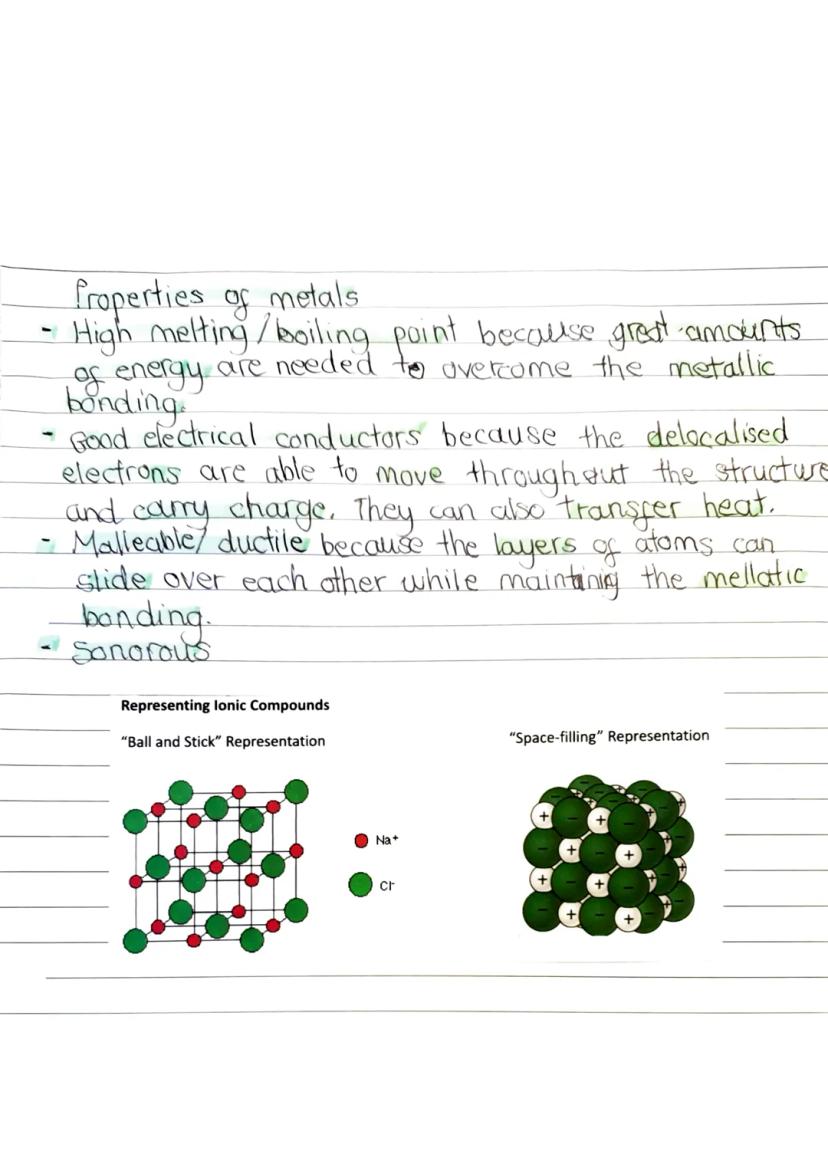
These oppositely charged ions attract each other through electrostatic forces, creating a giant lattice structure. This isn't just a few ions stuck together - it's a massive, regular pattern that continues in all directions throughout the entire crystal.
Scientific models help us visualise these complex structures in simpler ways, showing the key features without overwhelming detail.
Key Point: Metals always form positive ions, non-metals always form negative ions - opposites attract!