The particulate nature of matter forms the foundation of chemistry,... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
187
•
Dec 14, 2025
•
Cami Carbo
@camicarbo123
The particulate nature of matter forms the foundation of chemistry,... Show more
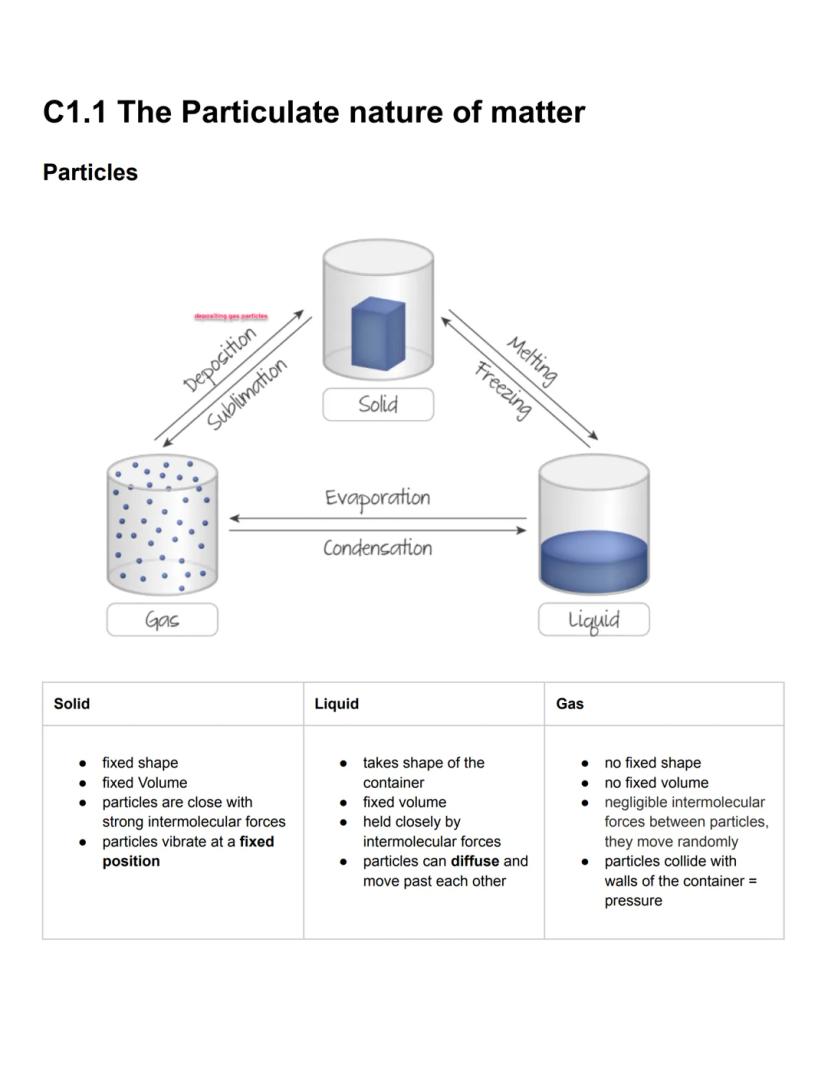





Matter exists in three main physical states, each with distinctive properties based on how particles are arranged. Solids have both fixed shape and volume because their particles are tightly packed with strong intermolecular forces, limiting them to vibrating in fixed positions. This explains why solids maintain their shape regardless of container.
Liquids take the shape of their container while maintaining a fixed volume. Their particles are held together by moderate intermolecular forces, allowing them to move past one another and diffuse. This property explains why liquids flow but don't expand to fill available space.
Gases have neither fixed shape nor volume, with particles moving randomly with negligible forces between them. Gas particles collide with container walls, creating pressure. Phase changes like melting, freezing, evaporation, condensation, sublimation, and deposition occur when matter transitions between these states.
Remember this! The strength of intermolecular forces determines the state of matter - strongest in solids, moderate in liquids, and weakest in gases.
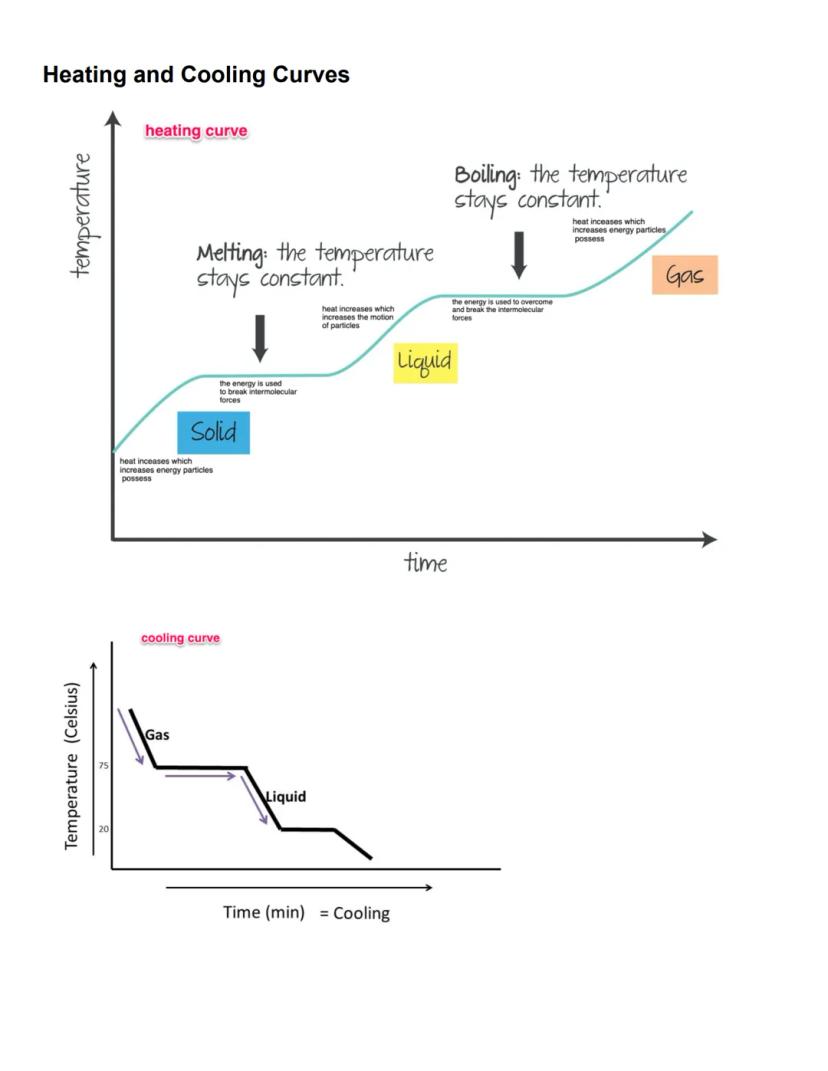
Heating and cooling curves visually represent what happens to substances as they change phase. On a heating curve, the temperature rises steadily as energy increases the motion of particles in a solid, until it reaches its melting point.
During melting, the temperature remains constant while heat energy works to overcome intermolecular forces, converting solid to liquid. Once melting is complete, temperature rises again until the boiling point is reached, where it plateaus again as energy breaks the remaining intermolecular forces to form a gas.
Cooling curves show the reverse process. As a gas cools, its temperature decreases until it reaches condensation, where temperature holds steady as gas becomes liquid. Further cooling eventually leads to freezing, with another temperature plateau as liquid transforms to solid.
Quick tip! Flat portions of heating/cooling curves always represent phase changes, where energy is being used to break or form intermolecular bonds rather than change temperature.
Density is the mass per unit volume of a substance , typically measured in g/dm³. Objects with higher densities feel heavier than those with lower densities when comparing the same volume, which explains why a small lead weight feels heavier than a much larger piece of foam.
An element is the smallest part of an atom, while a compound consists of multiple elements chemically bonded together in fixed ratios. Compounds have different physical and chemical properties from their component elements—water behaves nothing like hydrogen or oxygen alone!
Mixtures contain substances that aren't chemically bonded, so they retain their individual properties. Heterogeneous mixtures have non-uniform composition with visible boundaries between phases (like oil and water), while homogeneous mixtures have uniform composition throughout (like salt dissolved in water).
Fascinating fact! Crude oil is a complex homogeneous mixture of hydrocarbons that gets separated through fractional distillation. Components with lower boiling points rise higher in the fractionating tower where it's cooler, allowing us to separate useful products like petrol, diesel, and kerosene.

Chemical reactions form the basis of countless processes in our world. The key property of any chemical reaction is that new substances are formed with different properties than the reactants. This happens because bonds in reactants break and new bonds form in products, resulting in energy changes.
The conservation of mass theory tells us there's a fixed relationship between reactants and products, so no mass is gained or lost during reactions. This fundamental principle requires us to balance chemical equations, ensuring the same number of atoms appear on both sides.
When writing chemical equations, we use state symbols to indicate the physical state of substances: (s) for solids, (l) for liquids, (g) for gases, and (aq) for aqueous solutions (dissolved in water). These symbols provide crucial information about the conditions of the reaction.
Key insight! Every chemical reaction involves energy changes—either releasing energy (exothermic) or absorbing it (endothermic)—as bonds break and form.
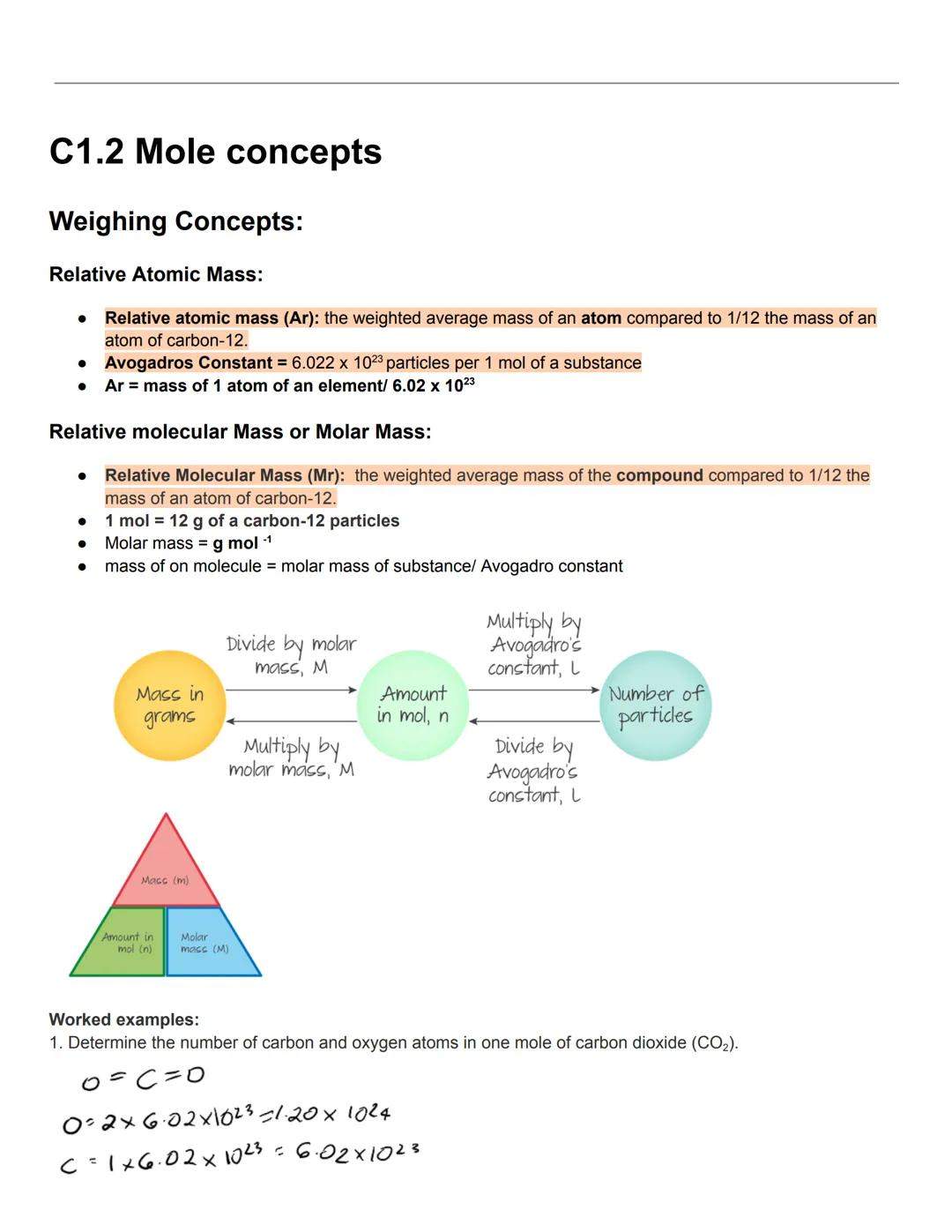
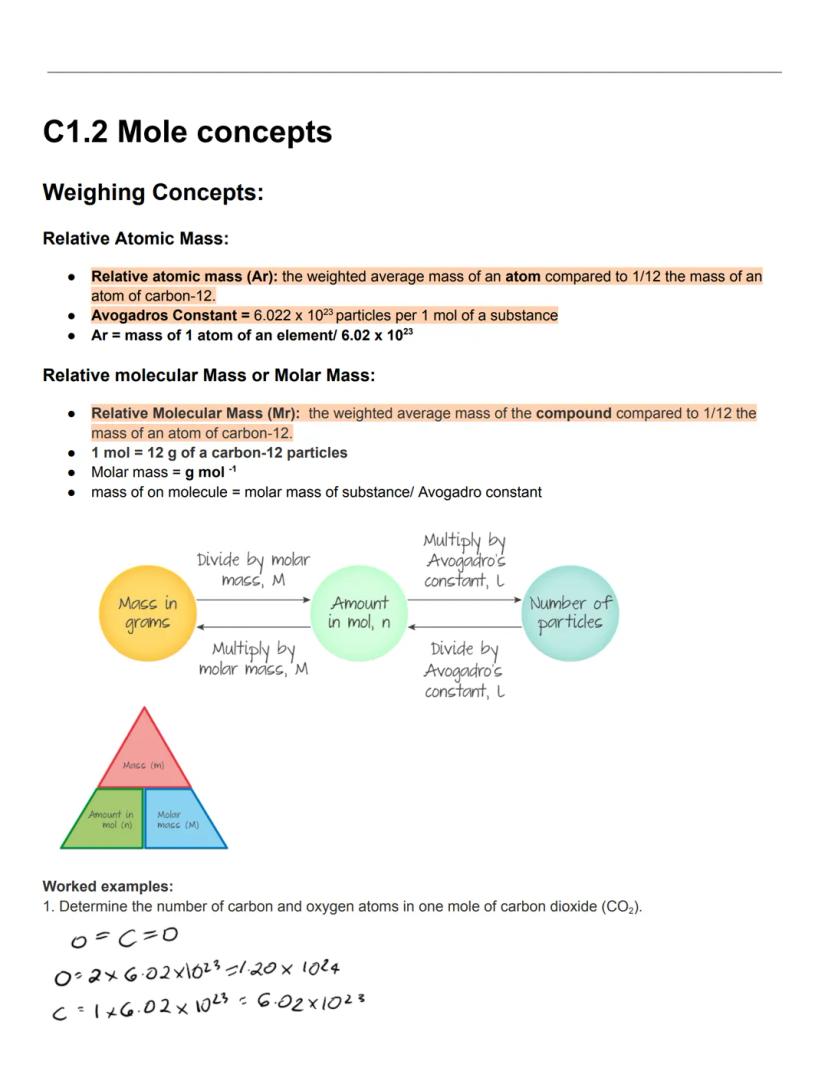
The mole is chemistry's counting unit, making it possible to work with the incredibly small particles in chemical reactions. Relative atomic mass (Ar) is the weighted average mass of an atom compared to 1/12 the mass of a carbon-12 atom. This gives us a practical way to compare elements.
Avogadro's constant (6.022 × 10²³) tells us exactly how many particles are in one mole of any substance. This means one mole of carbon-12 has a mass of exactly 12g, and one mole of any element has a mass in grams equal to its relative atomic mass.
Relative molecular mass (Mr) or molar mass works similarly for compounds—it's the weighted average mass of the compound compared to 1/12 the mass of carbon-12. The units are g/mol, and you can calculate it by adding up the relative atomic masses of all atoms in the compound.
Mole magic! You can convert between mass, moles, and number of particles using these relationships: mass (g) = amount (mol) × molar mass , and number of particles = amount (mol) × Avogadro's constant.
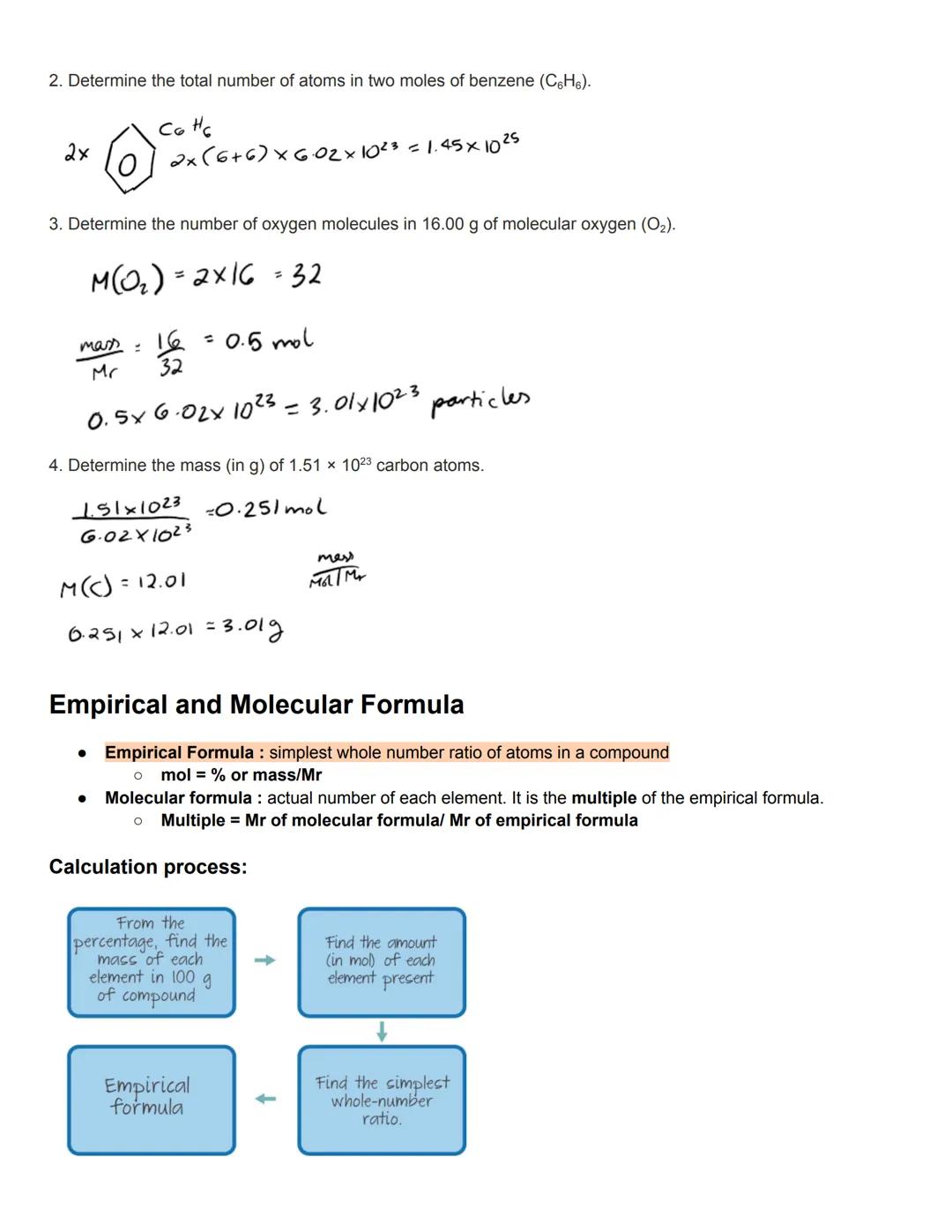
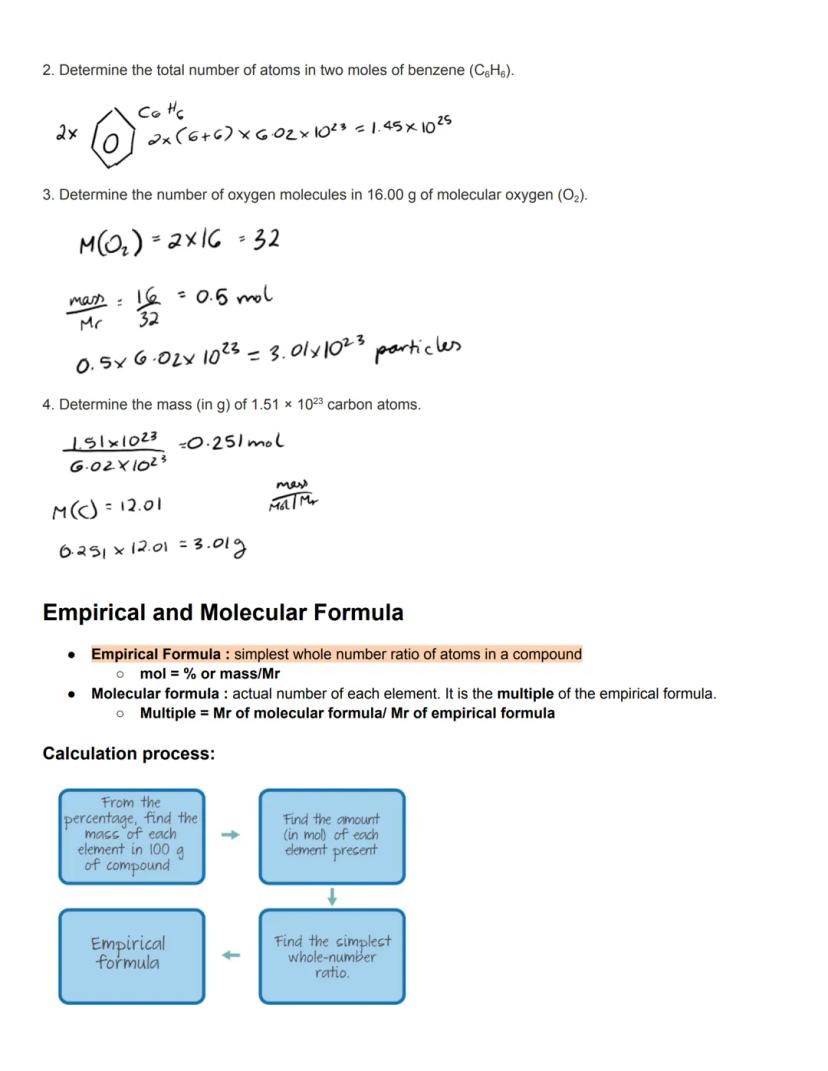
Empirical formulas show the simplest whole number ratio of atoms in a compound. To find this, you'll convert percentage composition or experimental data to moles, then find the simplest whole-number ratio between elements. This gives you a fundamental understanding of a compound's composition.
Molecular formulas reveal the actual number of each element in a molecule. They're often multiples of the empirical formula. To find the multiple, divide the molecular formula's Mr by the empirical formula's Mr. For example, the empirical formula of benzene is CH, but its molecular formula is C₆H₆.
Working with these formulas requires systematic calculation steps. First, convert your data to find the mass of each element, then convert to moles using the relative atomic masses. Finally, find the simplest whole-number ratio by dividing all values by the smallest number of moles.
Real-world application! Chemists use empirical formulas when developing new medicines to understand the basic composition of compounds before determining their complete molecular structure.
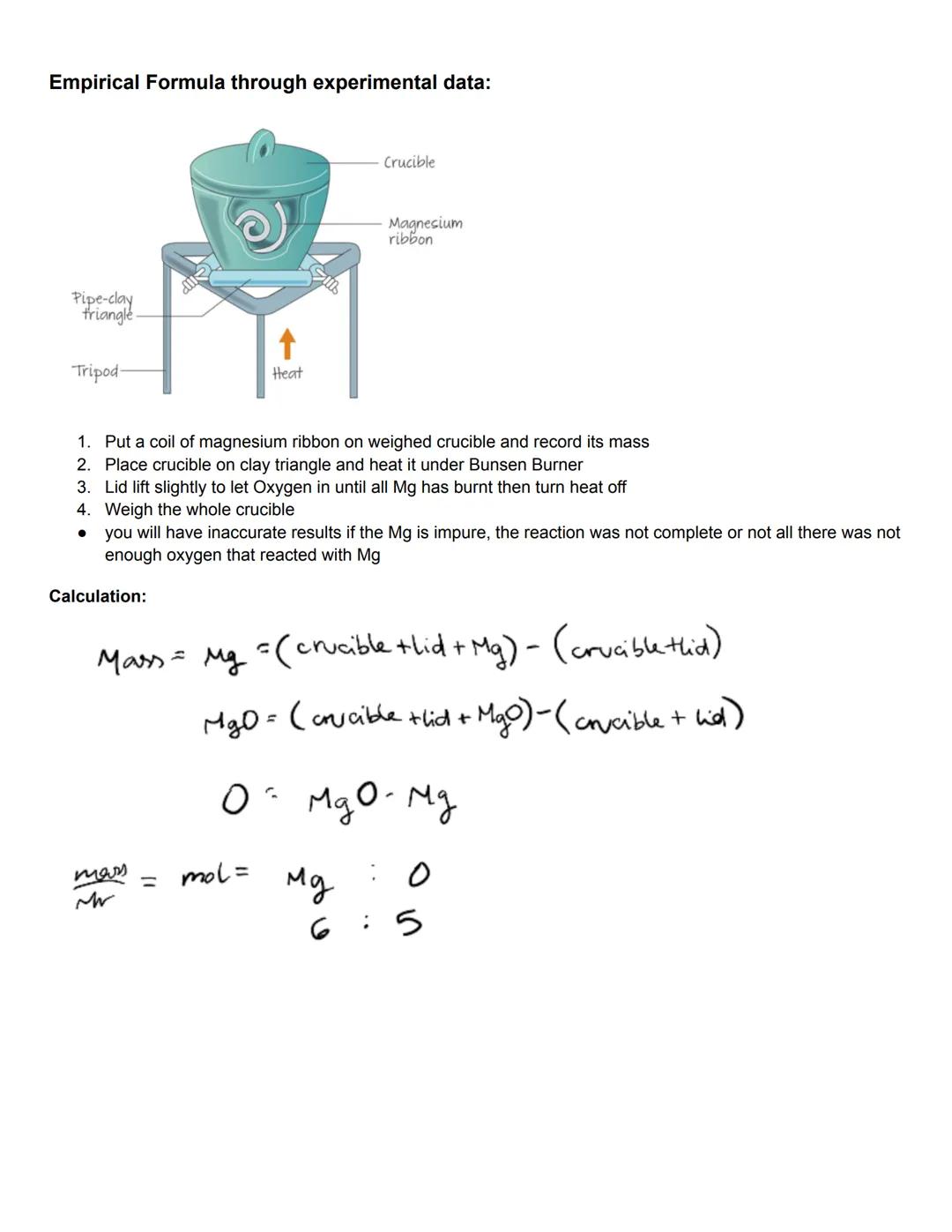
Experimental determination of empirical formulas often involves combustion or oxidation reactions. For example, to find the empirical formula of magnesium oxide, we can burn magnesium in oxygen and analyze the mass change. This hands-on approach connects theoretical concepts with practical chemistry.
The practical procedure involves weighing a magnesium ribbon, burning it completely in a crucible, and then reweighing. The difference in mass represents the oxygen that combined with the magnesium. From these masses, you can calculate the moles of each element and determine their ratio.
Accuracy in these experiments depends on several factors. If the magnesium is impure, the reaction isn't complete, or insufficient oxygen reacts with the metal, your results will be inaccurate. This highlights the importance of careful experimental technique in obtaining reliable data.
Pro tip! When calculating empirical formulas from experimental data, always record all masses to at least 3 decimal places to minimize percentage error in your final formula.

Combustion analysis is a powerful technique for determining the empirical formula of compounds containing carbon, hydrogen, and oxygen. When such compounds burn completely in excess oxygen, they produce carbon dioxide and water, which can be measured to calculate the composition of the original compound.
In a typical analysis, the masses of CO₂ and H₂O produced from burning a known mass of the compound are measured. From these masses, you can calculate the mass of carbon (from CO₂) and hydrogen (from H₂O). The oxygen content is determined by subtracting the masses of carbon and hydrogen from the original sample mass.
Once you have the masses of each element, convert them to moles by dividing by their respective relative atomic masses. Then find the simplest whole-number ratio to determine the empirical formula. This methodical approach allows chemists to identify unknown organic compounds.
Common mistake alert! When calculating the oxygen content in combustion analysis, students often forget that oxygen comes from both the original compound AND the oxygen used for combustion. Always calculate oxygen in the original compound by subtraction from the total mass.

In chemical reactions, the limiting reagent determines how much product can be formed. It's the reactant that's completely consumed first, while excess reactants remain after the reaction completes. Identifying the limiting reagent is crucial for calculating maximum possible yield from a reaction.
To determine the limiting reagent, convert masses of all reactants to moles and divide by their coefficients in the balanced equation. The reactant with the smallest resulting value is the limiting reagent. The theoretical yield is the maximum amount of product possible, assuming 100% conversion of the limiting reagent.
Avogadro's Law states that equal volumes of gases under the same conditions contain the same number of particles. This forms the foundation of gas laws that describe relationships between pressure, volume, and temperature. Pressure and volume are inversely proportional (when one increases, the other decreases), while both pressure and volume are directly proportional to temperature.
Chemistry in action! Industrial chemical processes always calculate limiting reagents to maximize efficiency and minimize waste. This same principle applies whether you're making pharmaceuticals or baking a cake!

An ideal gas consists of particles that have negligible volume and no attractive forces between them. These particles move freely and collide elastically. While no real gas is truly ideal, gases approach ideal behavior at high temperatures and low pressures, where particles have enough energy to overcome any attractive forces.
The relationships between pressure, volume, and temperature for gases can be visualized through graphs. When pressure increases, volume decreases as particles have less space to move. When temperature increases, pressure increases due to more energetic collisions. Similarly, volume increases with temperature as particles collide with greater force.
Real gases deviate from ideal behavior because their particles do have some volume and experience attractive forces. These deviations become more significant at high pressures (where particle volume matters) and low temperatures (where attractive forces become more influential). Real gases typically have lower pressure than predicted by ideal gas laws due to these attractive forces.
Think about it: When you compress air in a bicycle pump, it gets noticeably warmer—this is the gas laws in action, as decreasing volume leads to increased pressure and temperature!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Cami Carbo
@camicarbo123
The particulate nature of matter forms the foundation of chemistry, explaining how solids, liquids, and gases behave based on their particle arrangements and forces. Understanding these concepts helps explain everyday phenomena like ice melting, water boiling, and chemical reactions.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
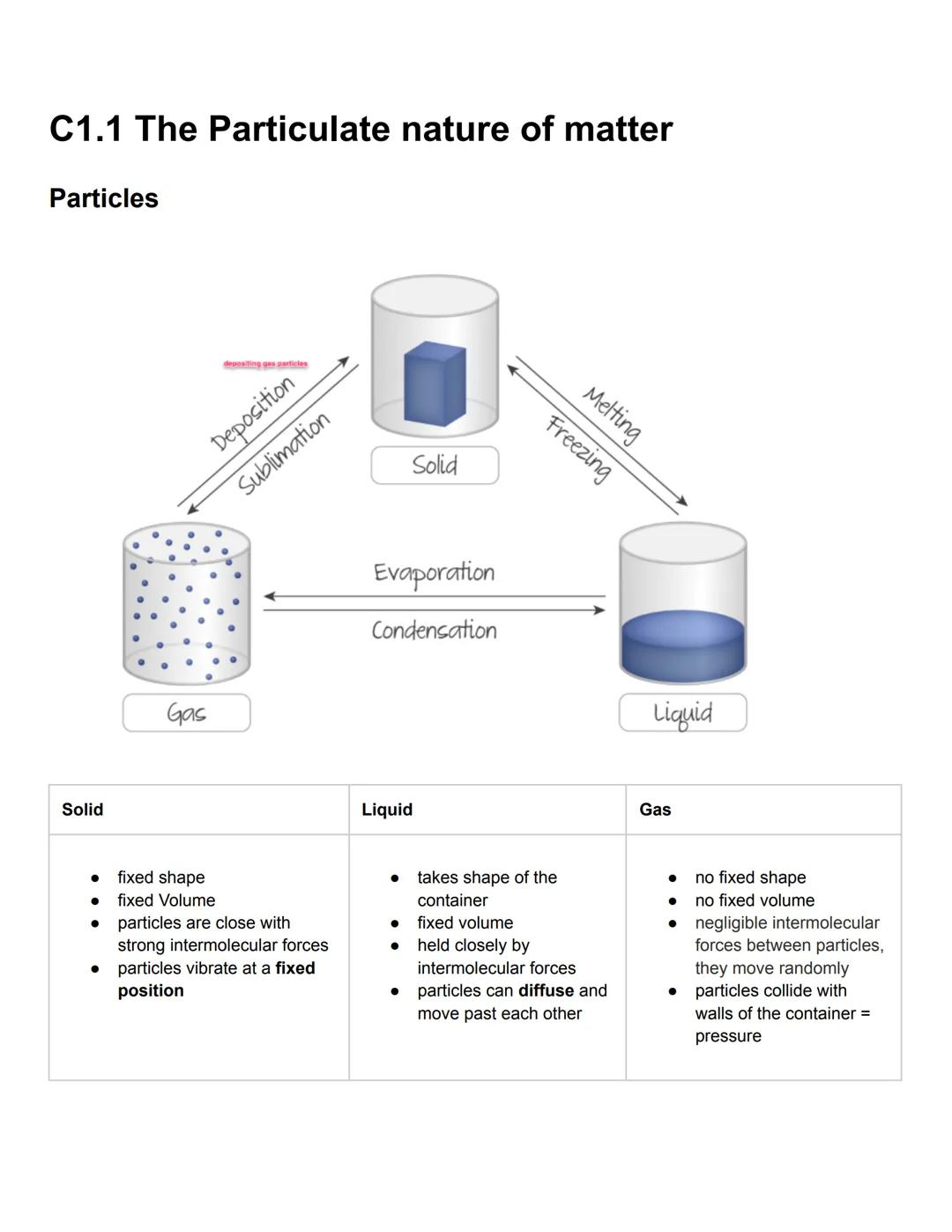
Matter exists in three main physical states, each with distinctive properties based on how particles are arranged. Solids have both fixed shape and volume because their particles are tightly packed with strong intermolecular forces, limiting them to vibrating in fixed positions. This explains why solids maintain their shape regardless of container.
Liquids take the shape of their container while maintaining a fixed volume. Their particles are held together by moderate intermolecular forces, allowing them to move past one another and diffuse. This property explains why liquids flow but don't expand to fill available space.
Gases have neither fixed shape nor volume, with particles moving randomly with negligible forces between them. Gas particles collide with container walls, creating pressure. Phase changes like melting, freezing, evaporation, condensation, sublimation, and deposition occur when matter transitions between these states.
Remember this! The strength of intermolecular forces determines the state of matter - strongest in solids, moderate in liquids, and weakest in gases.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
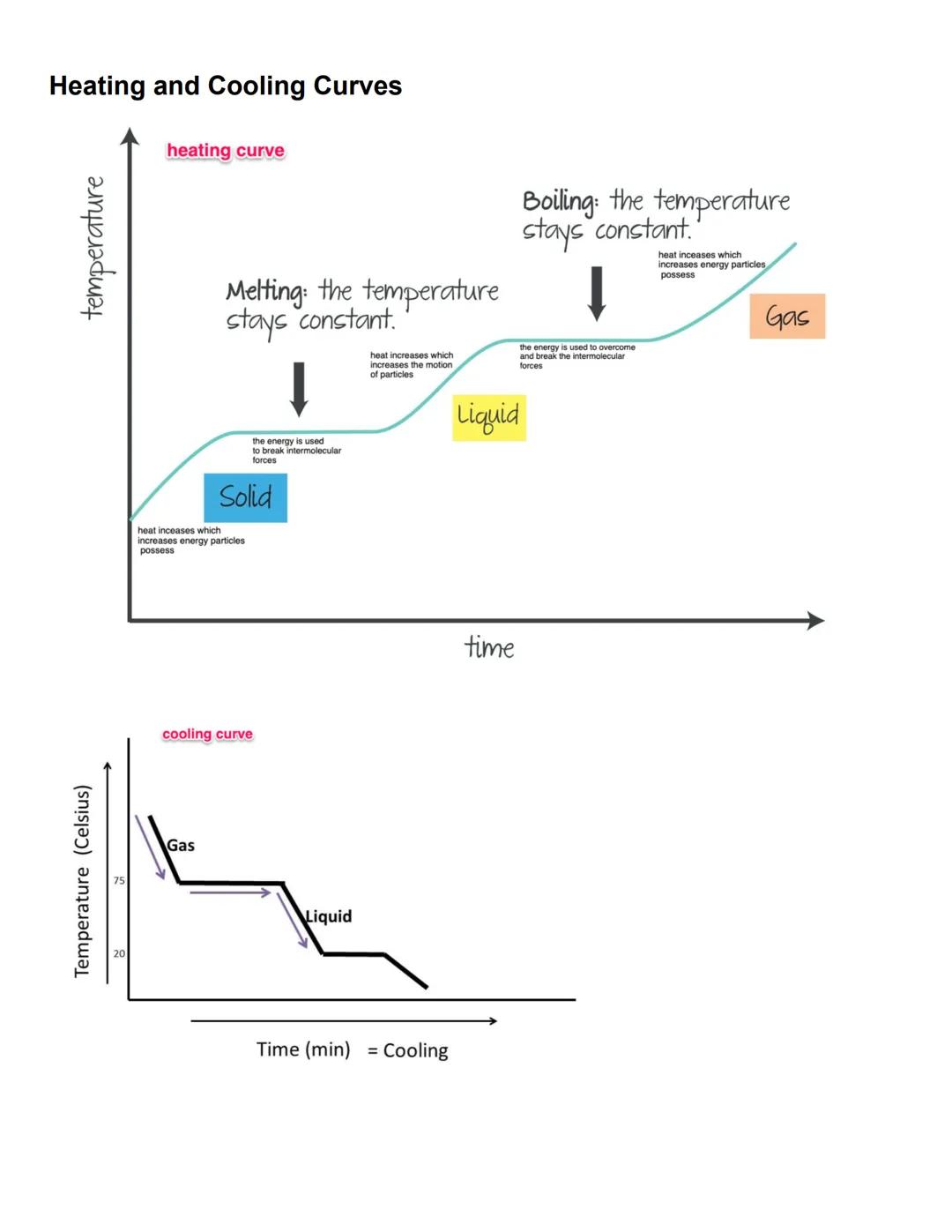
Heating and cooling curves visually represent what happens to substances as they change phase. On a heating curve, the temperature rises steadily as energy increases the motion of particles in a solid, until it reaches its melting point.
During melting, the temperature remains constant while heat energy works to overcome intermolecular forces, converting solid to liquid. Once melting is complete, temperature rises again until the boiling point is reached, where it plateaus again as energy breaks the remaining intermolecular forces to form a gas.
Cooling curves show the reverse process. As a gas cools, its temperature decreases until it reaches condensation, where temperature holds steady as gas becomes liquid. Further cooling eventually leads to freezing, with another temperature plateau as liquid transforms to solid.
Quick tip! Flat portions of heating/cooling curves always represent phase changes, where energy is being used to break or form intermolecular bonds rather than change temperature.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
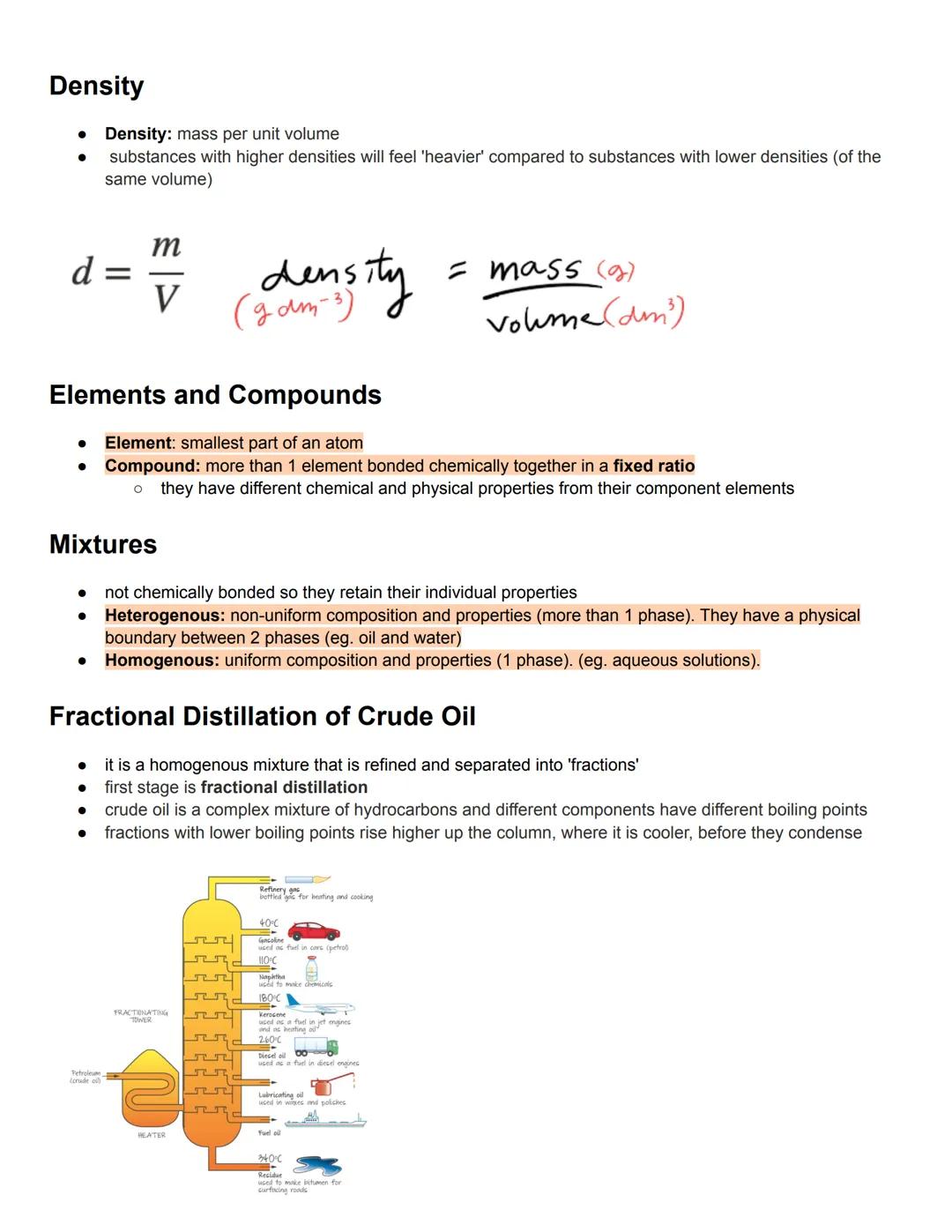
Density is the mass per unit volume of a substance , typically measured in g/dm³. Objects with higher densities feel heavier than those with lower densities when comparing the same volume, which explains why a small lead weight feels heavier than a much larger piece of foam.
An element is the smallest part of an atom, while a compound consists of multiple elements chemically bonded together in fixed ratios. Compounds have different physical and chemical properties from their component elements—water behaves nothing like hydrogen or oxygen alone!
Mixtures contain substances that aren't chemically bonded, so they retain their individual properties. Heterogeneous mixtures have non-uniform composition with visible boundaries between phases (like oil and water), while homogeneous mixtures have uniform composition throughout (like salt dissolved in water).
Fascinating fact! Crude oil is a complex homogeneous mixture of hydrocarbons that gets separated through fractional distillation. Components with lower boiling points rise higher in the fractionating tower where it's cooler, allowing us to separate useful products like petrol, diesel, and kerosene.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Chemical reactions form the basis of countless processes in our world. The key property of any chemical reaction is that new substances are formed with different properties than the reactants. This happens because bonds in reactants break and new bonds form in products, resulting in energy changes.
The conservation of mass theory tells us there's a fixed relationship between reactants and products, so no mass is gained or lost during reactions. This fundamental principle requires us to balance chemical equations, ensuring the same number of atoms appear on both sides.
When writing chemical equations, we use state symbols to indicate the physical state of substances: (s) for solids, (l) for liquids, (g) for gases, and (aq) for aqueous solutions (dissolved in water). These symbols provide crucial information about the conditions of the reaction.
Key insight! Every chemical reaction involves energy changes—either releasing energy (exothermic) or absorbing it (endothermic)—as bonds break and form.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The mole is chemistry's counting unit, making it possible to work with the incredibly small particles in chemical reactions. Relative atomic mass (Ar) is the weighted average mass of an atom compared to 1/12 the mass of a carbon-12 atom. This gives us a practical way to compare elements.
Avogadro's constant (6.022 × 10²³) tells us exactly how many particles are in one mole of any substance. This means one mole of carbon-12 has a mass of exactly 12g, and one mole of any element has a mass in grams equal to its relative atomic mass.
Relative molecular mass (Mr) or molar mass works similarly for compounds—it's the weighted average mass of the compound compared to 1/12 the mass of carbon-12. The units are g/mol, and you can calculate it by adding up the relative atomic masses of all atoms in the compound.
Mole magic! You can convert between mass, moles, and number of particles using these relationships: mass (g) = amount (mol) × molar mass , and number of particles = amount (mol) × Avogadro's constant.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Empirical formulas show the simplest whole number ratio of atoms in a compound. To find this, you'll convert percentage composition or experimental data to moles, then find the simplest whole-number ratio between elements. This gives you a fundamental understanding of a compound's composition.
Molecular formulas reveal the actual number of each element in a molecule. They're often multiples of the empirical formula. To find the multiple, divide the molecular formula's Mr by the empirical formula's Mr. For example, the empirical formula of benzene is CH, but its molecular formula is C₆H₆.
Working with these formulas requires systematic calculation steps. First, convert your data to find the mass of each element, then convert to moles using the relative atomic masses. Finally, find the simplest whole-number ratio by dividing all values by the smallest number of moles.
Real-world application! Chemists use empirical formulas when developing new medicines to understand the basic composition of compounds before determining their complete molecular structure.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
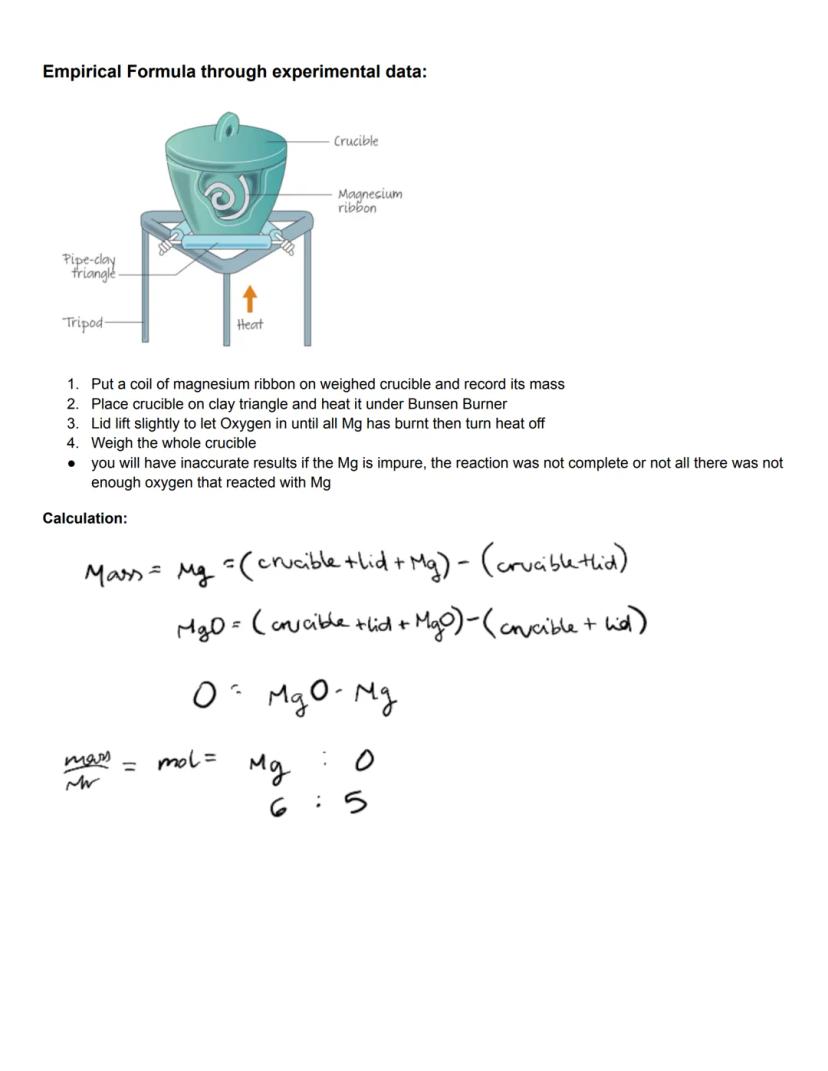
Experimental determination of empirical formulas often involves combustion or oxidation reactions. For example, to find the empirical formula of magnesium oxide, we can burn magnesium in oxygen and analyze the mass change. This hands-on approach connects theoretical concepts with practical chemistry.
The practical procedure involves weighing a magnesium ribbon, burning it completely in a crucible, and then reweighing. The difference in mass represents the oxygen that combined with the magnesium. From these masses, you can calculate the moles of each element and determine their ratio.
Accuracy in these experiments depends on several factors. If the magnesium is impure, the reaction isn't complete, or insufficient oxygen reacts with the metal, your results will be inaccurate. This highlights the importance of careful experimental technique in obtaining reliable data.
Pro tip! When calculating empirical formulas from experimental data, always record all masses to at least 3 decimal places to minimize percentage error in your final formula.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Combustion analysis is a powerful technique for determining the empirical formula of compounds containing carbon, hydrogen, and oxygen. When such compounds burn completely in excess oxygen, they produce carbon dioxide and water, which can be measured to calculate the composition of the original compound.
In a typical analysis, the masses of CO₂ and H₂O produced from burning a known mass of the compound are measured. From these masses, you can calculate the mass of carbon (from CO₂) and hydrogen (from H₂O). The oxygen content is determined by subtracting the masses of carbon and hydrogen from the original sample mass.
Once you have the masses of each element, convert them to moles by dividing by their respective relative atomic masses. Then find the simplest whole-number ratio to determine the empirical formula. This methodical approach allows chemists to identify unknown organic compounds.
Common mistake alert! When calculating the oxygen content in combustion analysis, students often forget that oxygen comes from both the original compound AND the oxygen used for combustion. Always calculate oxygen in the original compound by subtraction from the total mass.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
In chemical reactions, the limiting reagent determines how much product can be formed. It's the reactant that's completely consumed first, while excess reactants remain after the reaction completes. Identifying the limiting reagent is crucial for calculating maximum possible yield from a reaction.
To determine the limiting reagent, convert masses of all reactants to moles and divide by their coefficients in the balanced equation. The reactant with the smallest resulting value is the limiting reagent. The theoretical yield is the maximum amount of product possible, assuming 100% conversion of the limiting reagent.
Avogadro's Law states that equal volumes of gases under the same conditions contain the same number of particles. This forms the foundation of gas laws that describe relationships between pressure, volume, and temperature. Pressure and volume are inversely proportional (when one increases, the other decreases), while both pressure and volume are directly proportional to temperature.
Chemistry in action! Industrial chemical processes always calculate limiting reagents to maximize efficiency and minimize waste. This same principle applies whether you're making pharmaceuticals or baking a cake!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
An ideal gas consists of particles that have negligible volume and no attractive forces between them. These particles move freely and collide elastically. While no real gas is truly ideal, gases approach ideal behavior at high temperatures and low pressures, where particles have enough energy to overcome any attractive forces.
The relationships between pressure, volume, and temperature for gases can be visualized through graphs. When pressure increases, volume decreases as particles have less space to move. When temperature increases, pressure increases due to more energetic collisions. Similarly, volume increases with temperature as particles collide with greater force.
Real gases deviate from ideal behavior because their particles do have some volume and experience attractive forces. These deviations become more significant at high pressures (where particle volume matters) and low temperatures (where attractive forces become more influential). Real gases typically have lower pressure than predicted by ideal gas laws due to these attractive forces.
Think about it: When you compress air in a bicycle pump, it gets noticeably warmer—this is the gas laws in action, as decreasing volume leads to increased pressure and temperature!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
6
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the process of preparing a volumetric solution and conducting an acid-base titration to determine the concentration of sodium hydroxide. This practical guide covers essential laboratory techniques, chemical calculations, and safety precautions, ensuring a comprehensive understanding of titration methods. Ideal for chemistry students seeking to master titration skills and laboratory practices.
grade 7+ gcse combined science notes
Explore essential laboratory techniques in chemical analysis, including stoichiometric calculations, gravimetric analysis, and the use of various chemical apparatus such as conical flasks and digital balances. This summary provides insights into acid-base titrations and methods for improving percentage yield in reactions.
Explore essential laboratory techniques in Higher Chemistry, focusing on titration methods, standard solutions, and the calculation of unknown concentrations. This summary covers key concepts such as equivalence points, chemical principles, and practical applications in chemical analysis. Ideal for students preparing for exams or seeking to enhance their understanding of chemical processes.
Explore essential concepts in A Level Chemistry, including mole calculations, Avogadro's constant, atomic structure, and empirical formulas. This resource provides clear explanations and key formulas to enhance your understanding of chemical principles. Ideal for exam preparation and mastering foundational topics in chemistry.
Explore essential mole calculations and stoichiometry concepts for A-Level Chemistry. This study resource covers the amount of substance, back titration methods, and detailed examples to enhance your understanding of chemical calculations. Perfect for exam preparation and mastering key concepts.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user