Ever wondered why some reactions happen instantly whilst others take... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
4
0
Nona
12/8/2025
Chemistry
Rates of Reaction
217
•
Dec 8, 2025
•
Ever wondered why some reactions happen instantly whilst others take... Show more

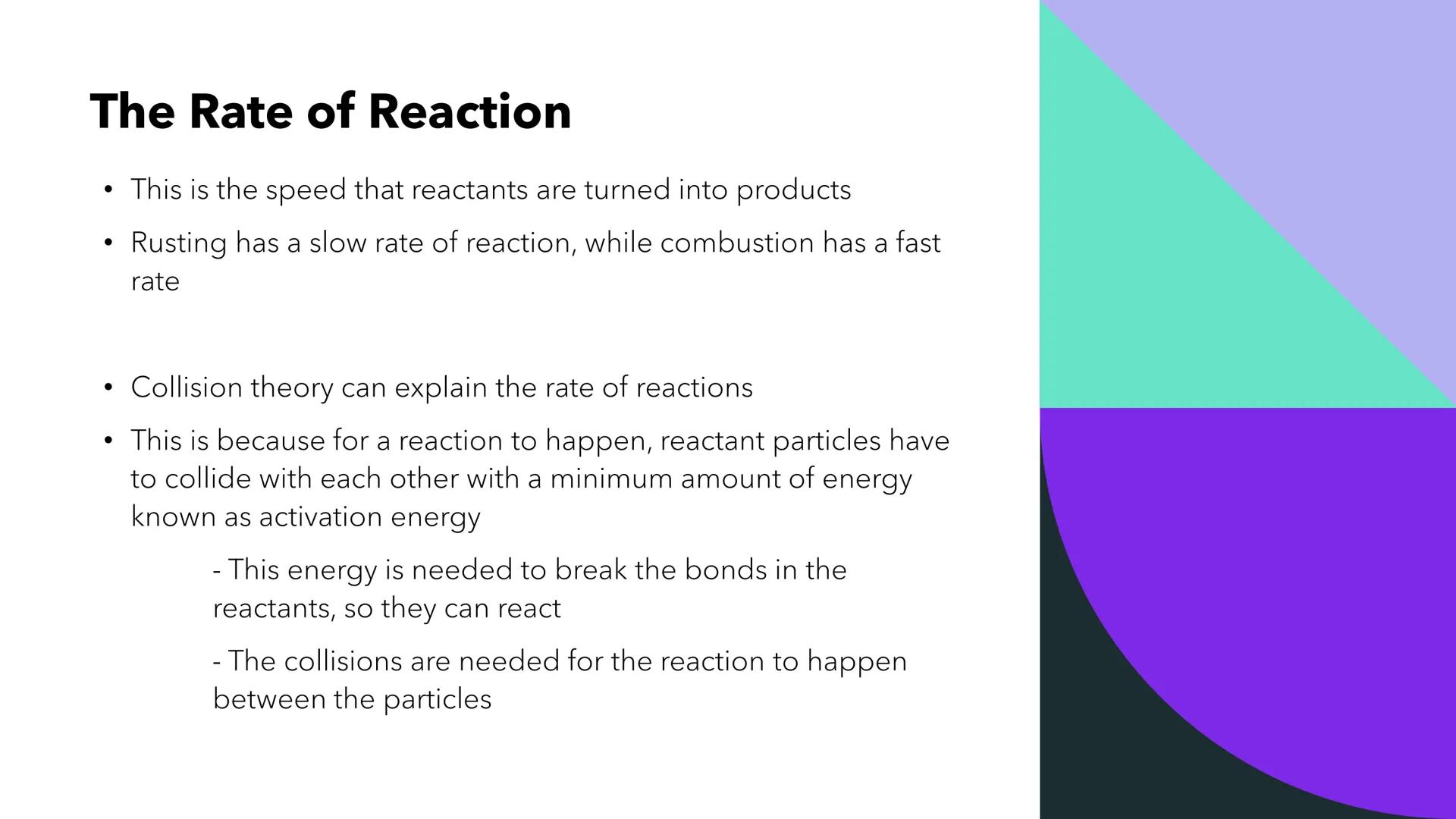
Think of rate of reaction as the speed at which your reactants transform into products - it's that simple! Rusting happens slowly over months, but lighting a match creates combustion in seconds.
Collision theory explains why reactions happen at different speeds. For any reaction to occur, particles must crash into each other with enough force - this minimum energy needed is called activation energy.
Without enough activation energy, particles just bounce off each other like soft tennis balls. But with sufficient energy, bonds break and new products form, making successful reactions possible.
Quick Tip: Remember that ALL reactions need collisions AND enough energy - both conditions must be met!
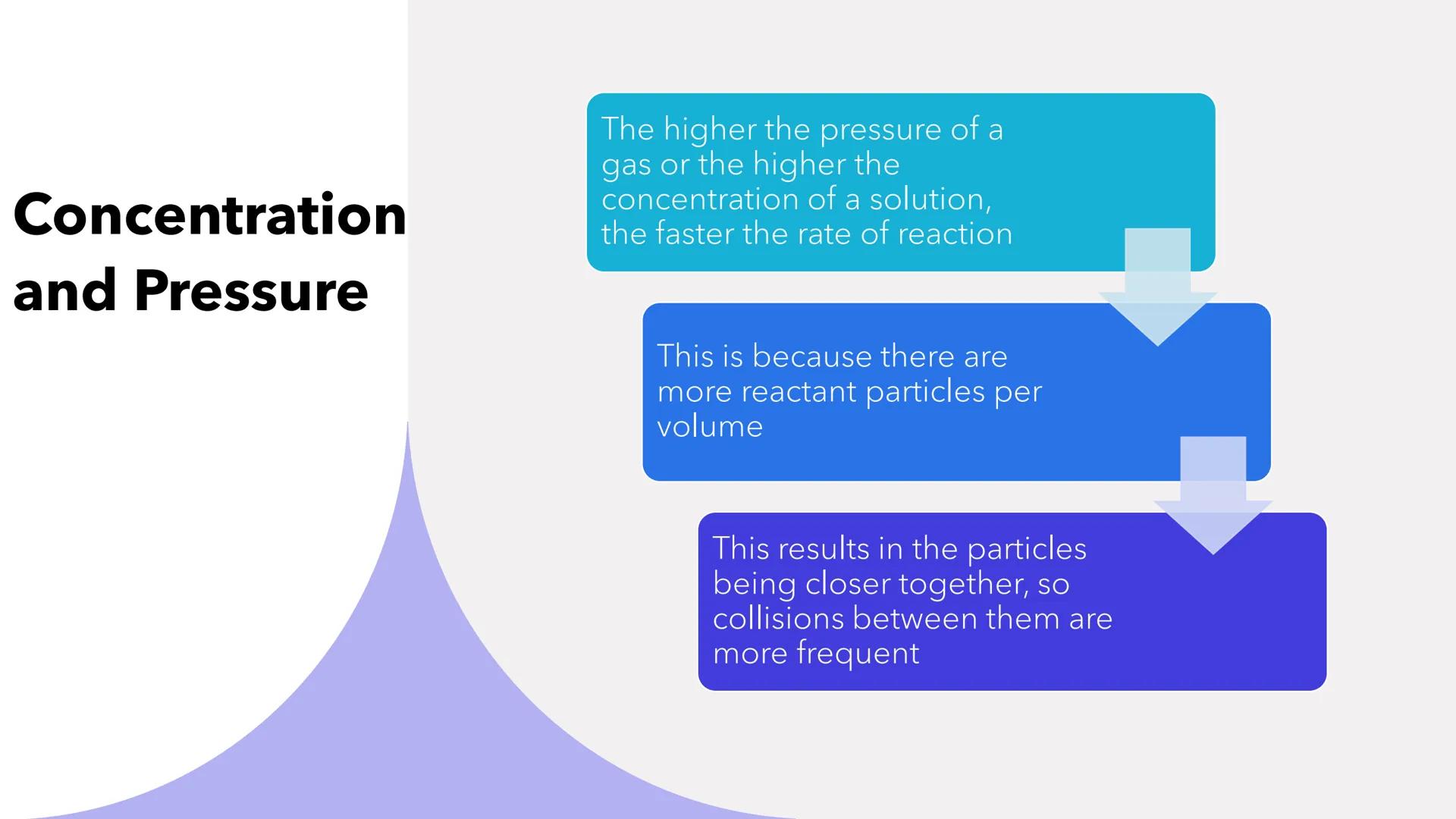
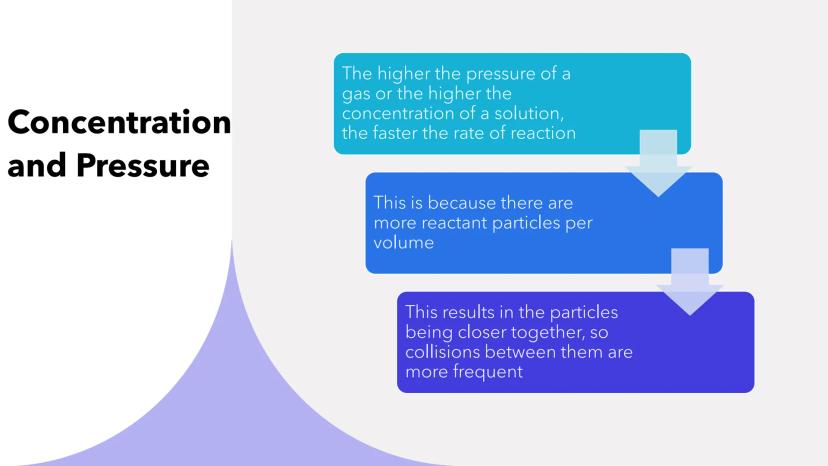
Higher concentration in solutions and higher pressure in gases both speed up reactions significantly. It's like having more people in a smaller room - collisions become inevitable!
When you increase concentration or pressure, you're cramming more reactant particles into the same space. This means particles are practically on top of each other, making frequent collisions much more likely.
Think of it this way: more particles per volume equals more opportunities for successful reactions to happen.
Real-world Connection: This is why pressurised spray cans work so effectively - the high pressure ensures rapid reactions!
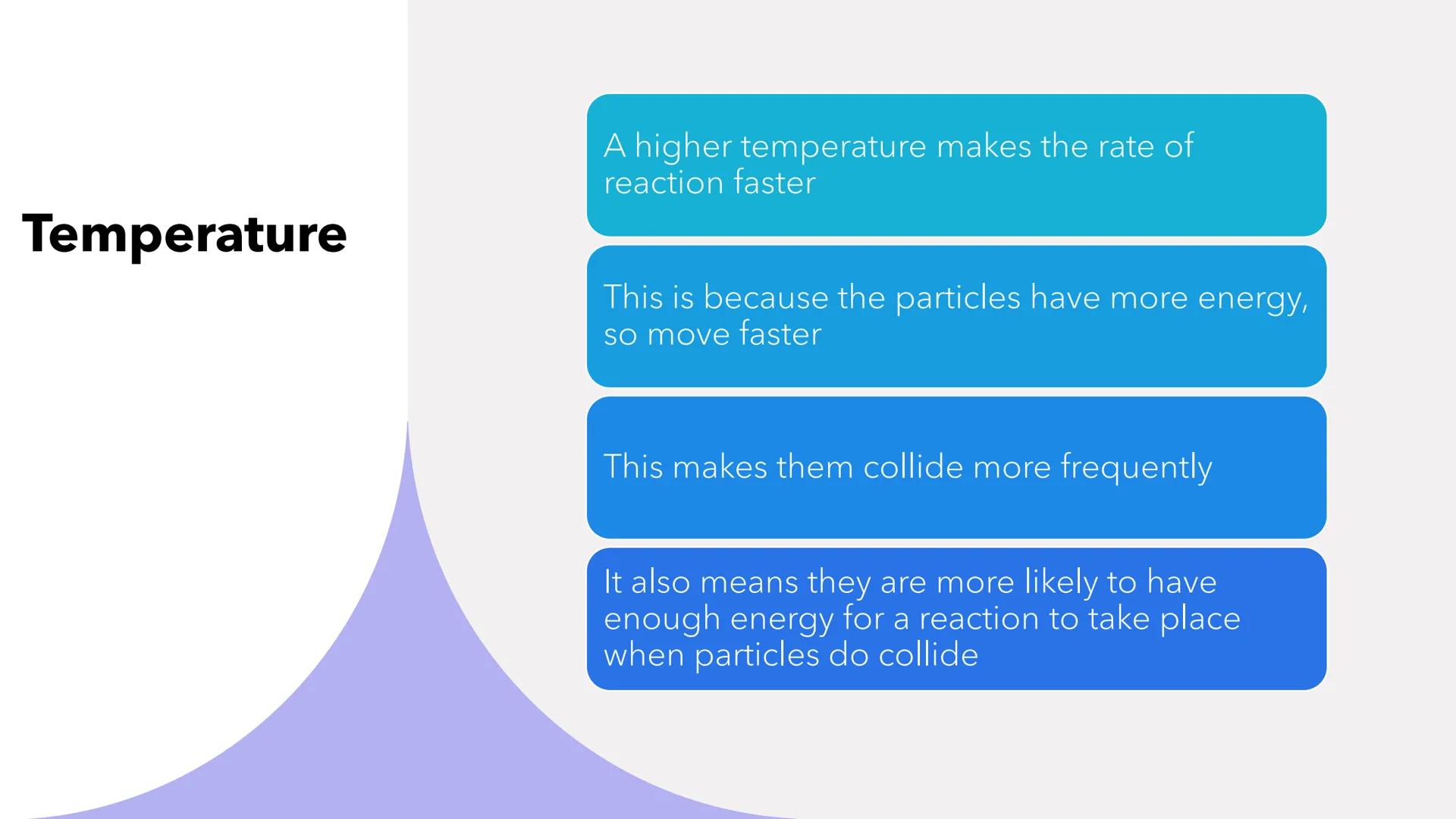
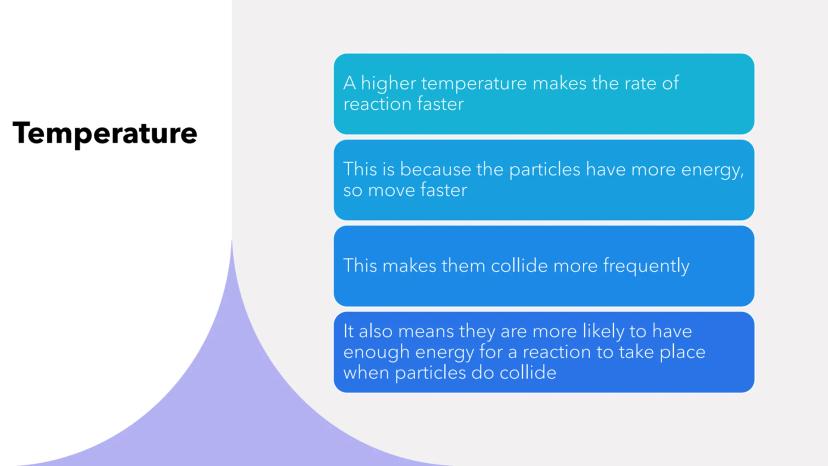
Cranking up the temperature is like giving particles an energy drink - they move faster and collide more often! Higher temperatures increase reaction rates in two brilliant ways.
First, speedy particles collide more frequently because they're zipping around with extra energy. Second, these energetic particles are more likely to have enough activation energy when they do collide.
It's a double win - more collisions that are also more successful. This explains why we cook food at high temperatures and why reactions slow down in cold conditions.
Memory Trick: Hot particles = fast movement = frequent successful collisions = faster reactions!

Surface area only matters when you've got a solid involved in your reaction, but when it does matter, it makes a huge difference! Reactions happen on the surface of solids, so more surface equals faster reactions.
Imagine trying to dissolve a whole sugar cube versus granulated sugar - the granules dissolve much quicker because there's more surface exposed to the water.
You can increase surface area by chopping, grinding, or crushing solids into smaller pieces. Each cut creates more surface for particles to collide with simultaneously.
Practical Tip: This is why powdered medicines work faster than tablets - maximum surface area for quick absorption!
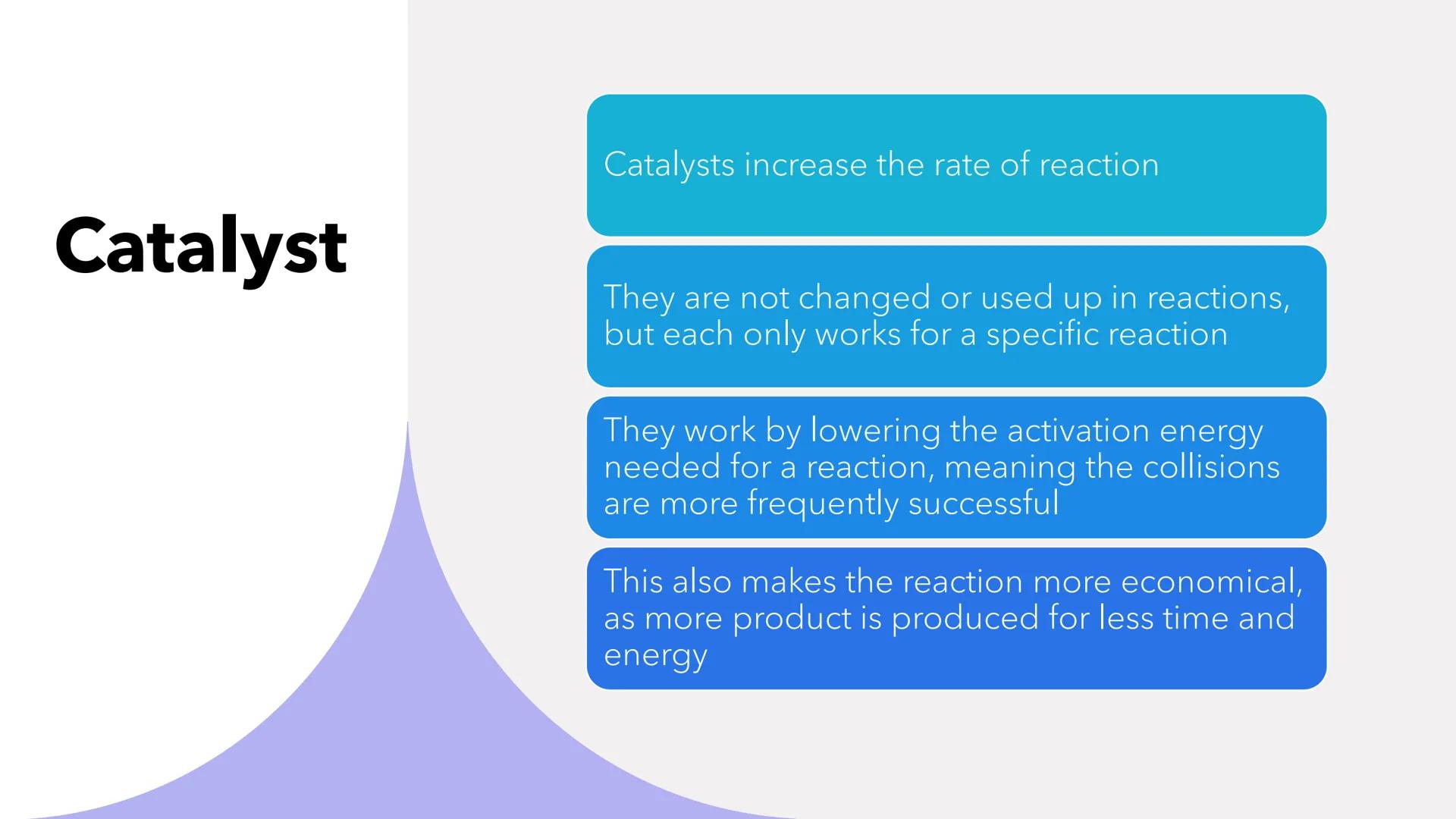
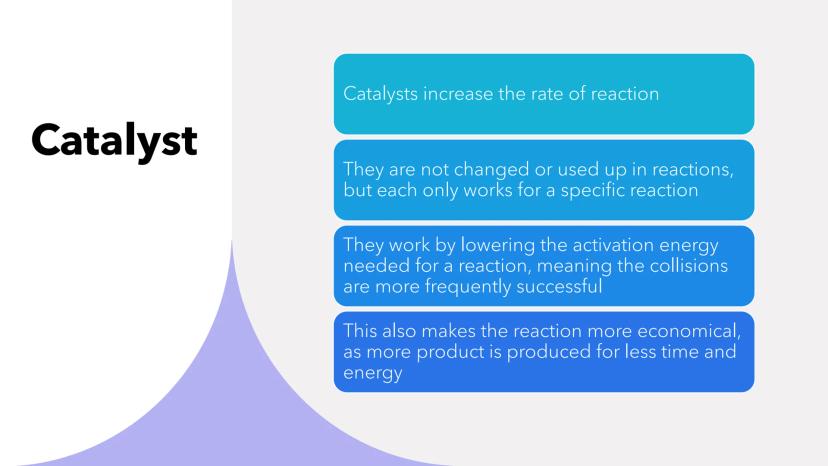
Catalysts are like the ultimate reaction helpers - they speed things up without getting used up themselves! Each catalyst is picky though, only working for specific reactions.
The clever bit is how they work: catalysts lower the activation energy needed for successful collisions. It's like lowering the height of a hurdle - suddenly more particles can "jump over" and react successfully.
This makes reactions faster AND more economical since you get more product in less time using less energy. Plus, since catalysts aren't consumed, you can reuse them repeatedly.
Exam Gold: Remember that catalysts are unchanged at the end - they're facilitators, not participants in the reaction!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
Quotes from every main character
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Ever wondered why some reactions happen instantly whilst others take ages? Understanding rates of reaction is crucial for your chemistry studies and explains everything from why food spoils to how industrial processes work efficiently.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Think of rate of reaction as the speed at which your reactants transform into products - it's that simple! Rusting happens slowly over months, but lighting a match creates combustion in seconds.
Collision theory explains why reactions happen at different speeds. For any reaction to occur, particles must crash into each other with enough force - this minimum energy needed is called activation energy.
Without enough activation energy, particles just bounce off each other like soft tennis balls. But with sufficient energy, bonds break and new products form, making successful reactions possible.
Quick Tip: Remember that ALL reactions need collisions AND enough energy - both conditions must be met!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Higher concentration in solutions and higher pressure in gases both speed up reactions significantly. It's like having more people in a smaller room - collisions become inevitable!
When you increase concentration or pressure, you're cramming more reactant particles into the same space. This means particles are practically on top of each other, making frequent collisions much more likely.
Think of it this way: more particles per volume equals more opportunities for successful reactions to happen.
Real-world Connection: This is why pressurised spray cans work so effectively - the high pressure ensures rapid reactions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Cranking up the temperature is like giving particles an energy drink - they move faster and collide more often! Higher temperatures increase reaction rates in two brilliant ways.
First, speedy particles collide more frequently because they're zipping around with extra energy. Second, these energetic particles are more likely to have enough activation energy when they do collide.
It's a double win - more collisions that are also more successful. This explains why we cook food at high temperatures and why reactions slow down in cold conditions.
Memory Trick: Hot particles = fast movement = frequent successful collisions = faster reactions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Surface area only matters when you've got a solid involved in your reaction, but when it does matter, it makes a huge difference! Reactions happen on the surface of solids, so more surface equals faster reactions.
Imagine trying to dissolve a whole sugar cube versus granulated sugar - the granules dissolve much quicker because there's more surface exposed to the water.
You can increase surface area by chopping, grinding, or crushing solids into smaller pieces. Each cut creates more surface for particles to collide with simultaneously.
Practical Tip: This is why powdered medicines work faster than tablets - maximum surface area for quick absorption!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Catalysts are like the ultimate reaction helpers - they speed things up without getting used up themselves! Each catalyst is picky though, only working for specific reactions.
The clever bit is how they work: catalysts lower the activation energy needed for successful collisions. It's like lowering the height of a hurdle - suddenly more particles can "jump over" and react successfully.
This makes reactions faster AND more economical since you get more product in less time using less energy. Plus, since catalysts aren't consumed, you can reuse them repeatedly.
Exam Gold: Remember that catalysts are unchanged at the end - they're facilitators, not participants in the reaction!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
4
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
notes include: - formula for rate of reaction - how to calculate rate of reaction graphically - illustrations, tables, graphs - highlighted important info - how to draw a tangent - worked examples
Learn about the reactivity and properties of metals, alkali metals, alkaline earth metals, and transition metals in the periodic table.
This note contains the summary of the topics redox reaction, electrochemistry, nuclear chemistry, and stoichiometry.
Learn about Henry Moseley's contribution to the modern periodic table and the arrangement of elements based on atomic number and properties.
ionic and covalent compounds
An introduction to chemistry including the different branches of chemistry.
Quotes from every main character
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user