Gibbs Free Energy is a fundamental concept in thermodynamics that... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
63
•
Feb 3, 2026
•
Gibbs Free Energy is a fundamental concept in thermodynamics that... Show more
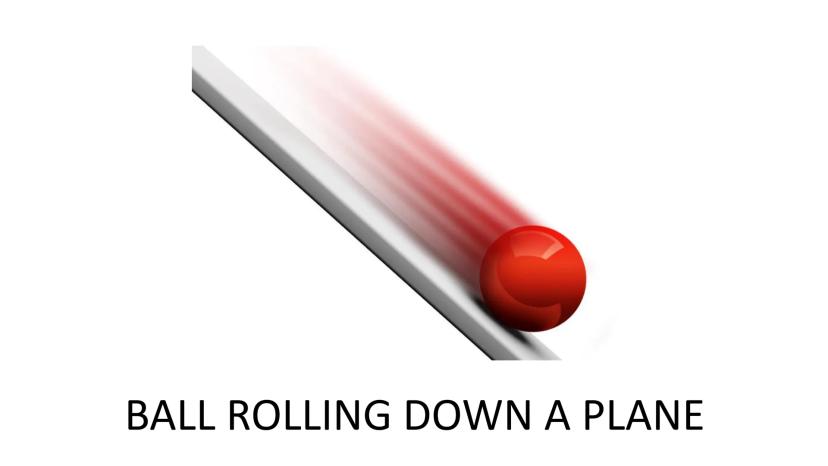





When a ball rolls down an inclined plane, it demonstrates energy conversion principles related to Gibbs Free Energy. The ball naturally moves from a position of higher potential energy to lower potential energy.
This spontaneous movement happens without requiring any additional energy input. In fact, energy is released during this process, similar to how exergonic chemical reactions release energy.
Think of this as a simple model for understanding why some chemical reactions happen on their own while others need energy to get started!
Quick Insight: Spontaneous processes like a rolling ball always move toward states of lower energy - this same principle applies to chemical reactions!
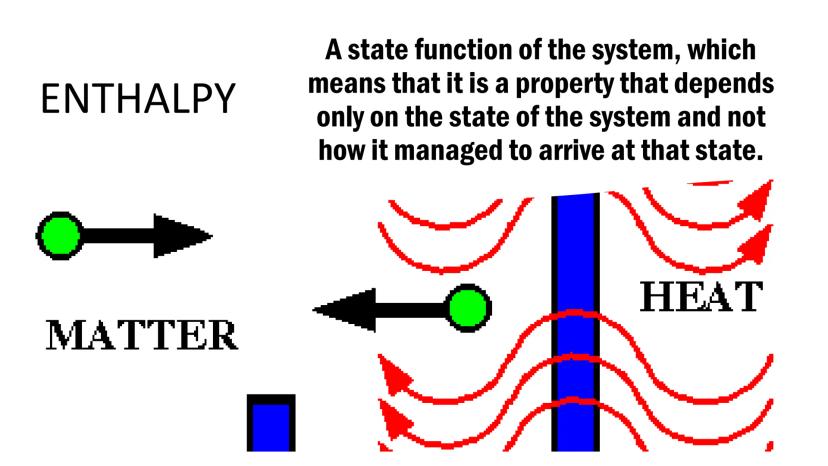
Enthalpy is a state function of a system, meaning it depends only on the current state of the system, not on how it got there. It's like checking your bank balance - the amount matters, not how the money got there.
Enthalpy relates to the heat content of matter. When chemical reactions occur, bonds break and form, causing either the release of heat (exothermic) or the absorption of heat (endothermic).
Understanding enthalpy helps us track energy flow during chemical changes. When you feel warmth from a hand warmer or cold from an ice pack, you're experiencing enthalpy changes in action!
Remember: Enthalpy (H) tracks heat energy in a system, and its changes (ΔH) tell us whether reactions release or absorb heat.
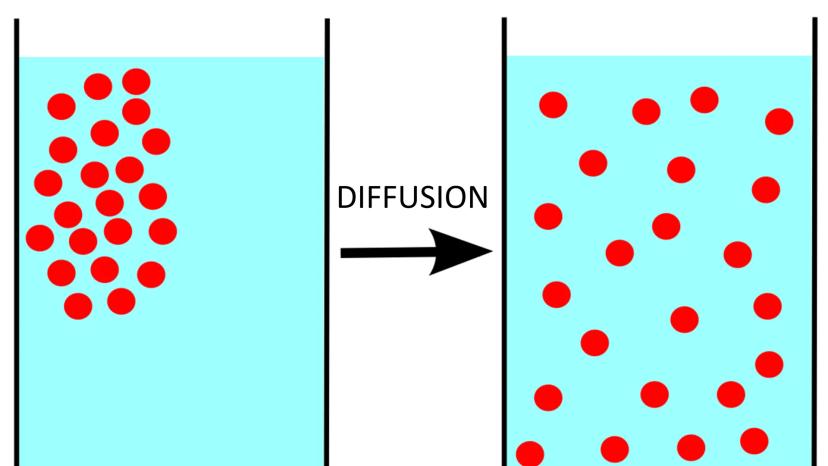
Diffusion occurs when particles spread from areas of high concentration to low concentration. It's like how the smell of perfume gradually fills a room.
This spontaneous process happens because particles naturally move toward more disordered arrangements. The movement increases overall entropy in the system without requiring energy input.
Diffusion is a perfect example of how systems naturally tend toward greater disorder. This tendency drives many processes in chemistry, biology, and even in everyday life!
Fun fact: Diffusion is responsible for how oxygen enters your bloodstream from your lungs and how nutrients enter your cells!
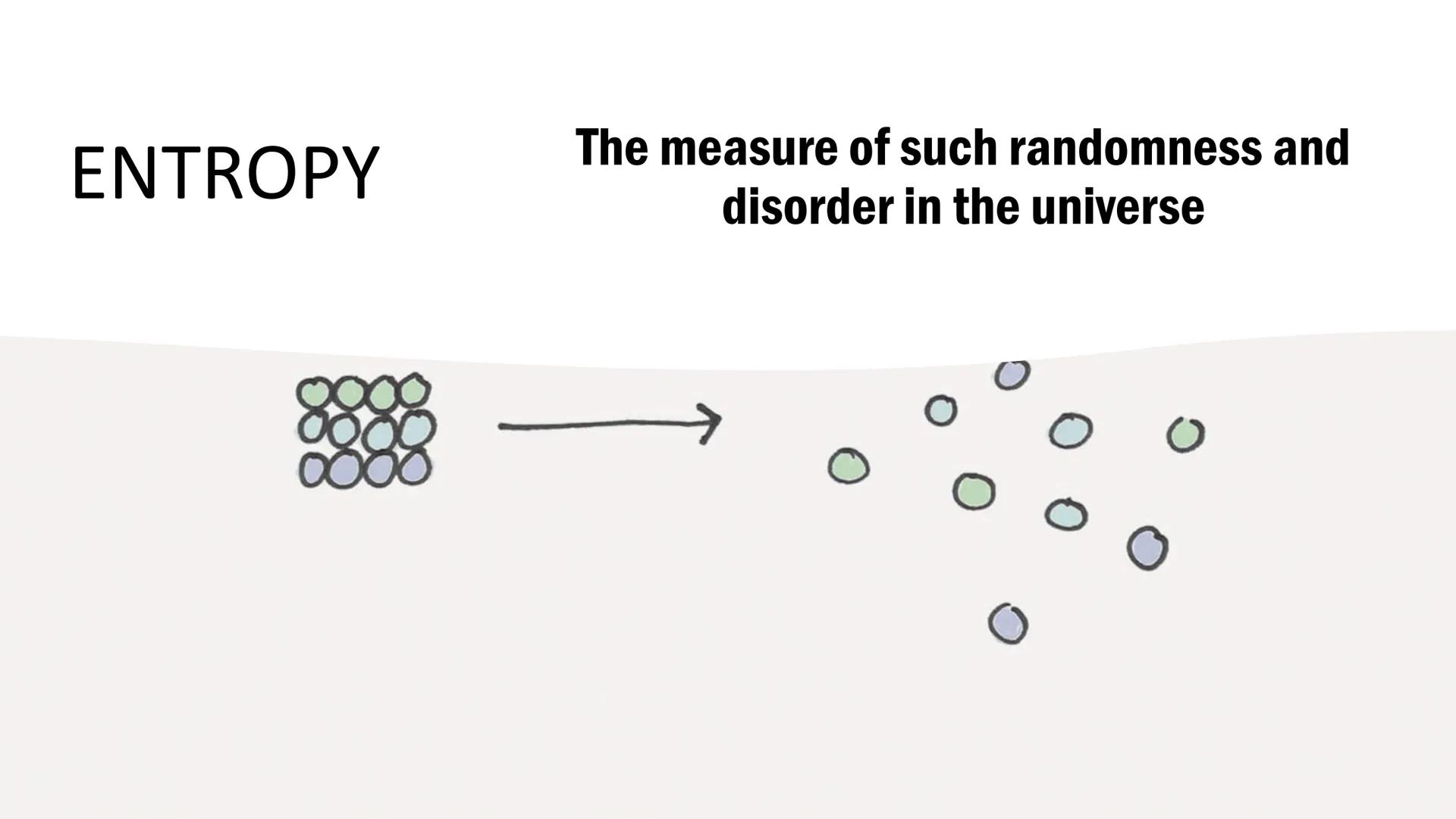
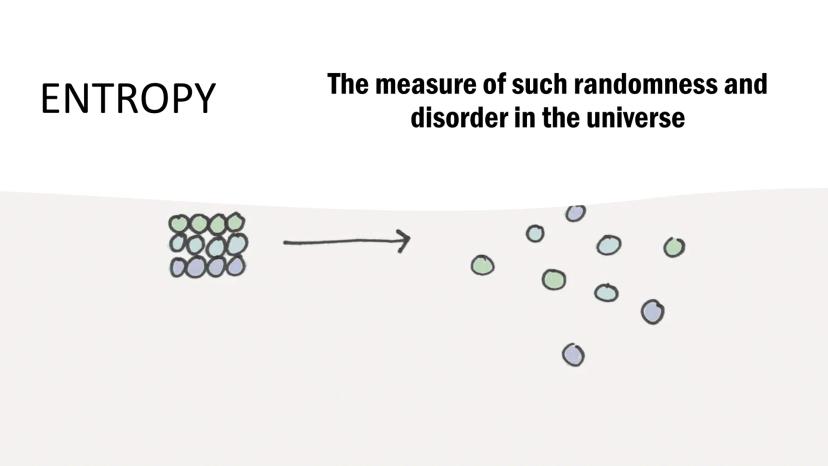
Entropy measures the randomness and disorder in the universe. Think of it as nature's tendency to become increasingly disorganized over time, like how a clean room naturally becomes messy.
Higher entropy means greater disorder. When a solid melts into a liquid or a liquid vaporizes into a gas, entropy increases because molecules move more freely and randomly.
Understanding entropy helps explain why some processes happen spontaneously. Nature always moves toward greater disorder unless energy is added to create order.
Quick tip: When you see the symbol S in equations, remember it's measuring how random or disordered a system is!
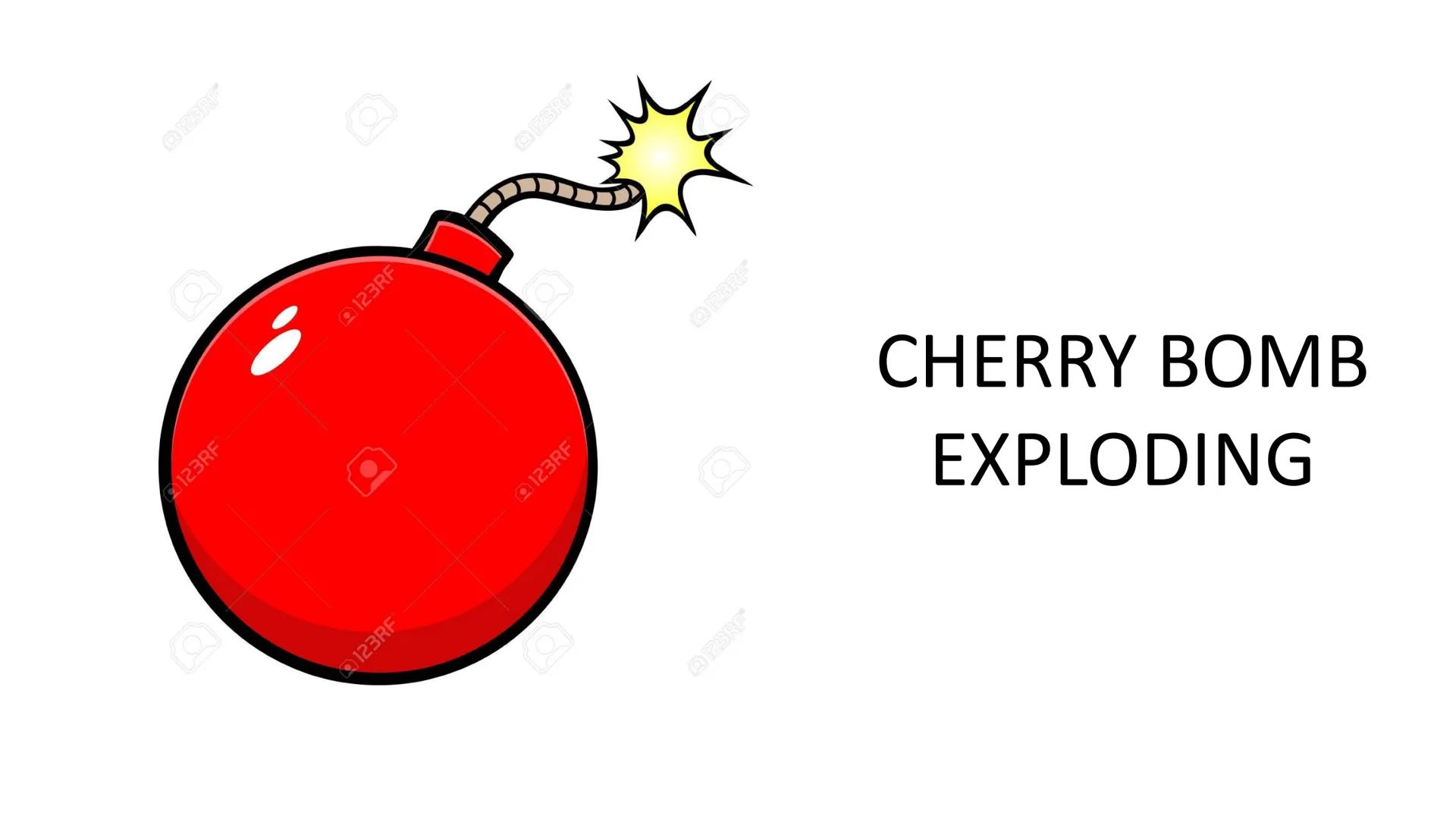
A cherry bomb explosion dramatically demonstrates entropy increase. The controlled, ordered arrangement of chemicals inside the firecracker transforms instantly into scattered gases, heat, and sound.
This explosive reaction releases stored chemical energy (negative ΔH) while dramatically increasing disorder (positive ΔS). Both factors make this process highly spontaneous with a very negative Gibbs Free Energy value.
The explosion can't be reversed naturally - you can't collect the scattered gases and energy to reassemble the cherry bomb. This illustrates how spontaneous processes with large entropy increases tend to be irreversible.
Safety note: While explosions perfectly demonstrate entropy principles, remember that firecrackers are dangerous and should only be handled by professionals!
Temperature is a measurement of how hot or cold something is on a defined scale. But in chemistry, it's much more than just a comfort reading—it's a crucial factor in determining reaction spontaneity.
Temperature directly affects the entropy term in the Gibbs Free Energy equation. As temperature increases, the impact of entropy (ΔS) becomes more significant in determining whether a reaction will occur spontaneously.
When using the Gibbs equation , temperature must be in Kelvin (K), not Celsius or Fahrenheit. Remember that 0°C = 273.15 K to make accurate calculations.
Interesting fact: Some reactions that aren't spontaneous at room temperature become spontaneous at higher temperatures because the TΔS term grows large enough to overcome a positive ΔH!

Gibbs Free Energy is the key thermodynamic concept that predicts whether chemical reactions will happen spontaneously. It combines both enthalpy and entropy effects into a single value.
When Gibbs Free Energy (ΔG) is negative, a reaction will occur on its own. When ΔG is positive, the reaction needs energy input to proceed. This simple rule helps chemists predict reaction behavior under specific conditions.
The beauty of Gibbs Free Energy is how it balances competing factors - the tendency to minimize energy (enthalpy) and maximize disorder (entropy). This balance explains why some endothermic reactions can still happen spontaneously!
Pro tip: The sign of ΔG is your quick indicator - negative means "go" and positive means "no" for spontaneous reactions.

Gibbs Free Energy measures the maximum reversible work a system can perform at constant temperature and pressure. It's like a chemical reaction's "budget" for doing useful work.
When a reaction has negative ΔG, it releases free energy that can be harnessed to do work. This explains why exergonic reactions can power processes in both laboratory settings and living organisms.
The remarkable thing about Gibbs Free Energy is that it combines two competing natural tendencies - minimizing energy and maximizing disorder - into a single predictive value that tells us what will happen in the real world.
Key insight: Think of Gibbs Free Energy as nature's way of determining which direction processes will flow spontaneously, just like water flows downhill.

Josiah Willard Gibbs developed what we now call Gibbs Free Energy in the 1870s. He initially termed it "available energy" in a system, highlighting its practical significance.
Gibbs was remarkably ahead of his time. His work on thermodynamics laid the foundation for modern chemical engineering, physical chemistry, and even quantum physics, though it wasn't widely recognized during his lifetime.
The concept of "available energy" perfectly captures the essence of Gibbs Free Energy - it's the energy in a system that's actually available to do useful work, not just the total energy present.
Historical note: Gibbs was the first American to earn a Ph.D. in engineering, and his contributions to science were so significant that Einstein called him "the greatest mind in American history."

The Gibbs Free Energy equation is expressed as: ΔG° = ΔH° - TΔS°. This elegantly combines heat energy (enthalpy), disorder (entropy), and temperature into one powerful predictive tool.
Each component plays a crucial role: ΔH° represents the heat released or absorbed, ΔS° tracks changes in randomness, and T (temperature in Kelvin) determines how much weight entropy carries in the equation.
The beauty of this equation is its predictive power - it tells you immediately if a reaction will happen spontaneously (when ΔG is negative) or requires energy input (when ΔG is positive).
Visualization tip: Picture the equation as a balance scale with ΔH on one side and TΔS on the other. Whichever side weighs more determines the sign of ΔG!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Gibbs Free Energy is a fundamental concept in thermodynamics that helps predict whether chemical reactions will occur spontaneously. It combines the effects of enthalpy (heat energy) and entropy (disorder) to determine if a reaction will happen on its own or... Show more

Access to all documents
Improve your grades
Join milions of students
When a ball rolls down an inclined plane, it demonstrates energy conversion principles related to Gibbs Free Energy. The ball naturally moves from a position of higher potential energy to lower potential energy.
This spontaneous movement happens without requiring any additional energy input. In fact, energy is released during this process, similar to how exergonic chemical reactions release energy.
Think of this as a simple model for understanding why some chemical reactions happen on their own while others need energy to get started!
Quick Insight: Spontaneous processes like a rolling ball always move toward states of lower energy - this same principle applies to chemical reactions!

Access to all documents
Improve your grades
Join milions of students
Enthalpy is a state function of a system, meaning it depends only on the current state of the system, not on how it got there. It's like checking your bank balance - the amount matters, not how the money got there.
Enthalpy relates to the heat content of matter. When chemical reactions occur, bonds break and form, causing either the release of heat (exothermic) or the absorption of heat (endothermic).
Understanding enthalpy helps us track energy flow during chemical changes. When you feel warmth from a hand warmer or cold from an ice pack, you're experiencing enthalpy changes in action!
Remember: Enthalpy (H) tracks heat energy in a system, and its changes (ΔH) tell us whether reactions release or absorb heat.

Access to all documents
Improve your grades
Join milions of students
Diffusion occurs when particles spread from areas of high concentration to low concentration. It's like how the smell of perfume gradually fills a room.
This spontaneous process happens because particles naturally move toward more disordered arrangements. The movement increases overall entropy in the system without requiring energy input.
Diffusion is a perfect example of how systems naturally tend toward greater disorder. This tendency drives many processes in chemistry, biology, and even in everyday life!
Fun fact: Diffusion is responsible for how oxygen enters your bloodstream from your lungs and how nutrients enter your cells!

Access to all documents
Improve your grades
Join milions of students
Entropy measures the randomness and disorder in the universe. Think of it as nature's tendency to become increasingly disorganized over time, like how a clean room naturally becomes messy.
Higher entropy means greater disorder. When a solid melts into a liquid or a liquid vaporizes into a gas, entropy increases because molecules move more freely and randomly.
Understanding entropy helps explain why some processes happen spontaneously. Nature always moves toward greater disorder unless energy is added to create order.
Quick tip: When you see the symbol S in equations, remember it's measuring how random or disordered a system is!

Access to all documents
Improve your grades
Join milions of students
A cherry bomb explosion dramatically demonstrates entropy increase. The controlled, ordered arrangement of chemicals inside the firecracker transforms instantly into scattered gases, heat, and sound.
This explosive reaction releases stored chemical energy (negative ΔH) while dramatically increasing disorder (positive ΔS). Both factors make this process highly spontaneous with a very negative Gibbs Free Energy value.
The explosion can't be reversed naturally - you can't collect the scattered gases and energy to reassemble the cherry bomb. This illustrates how spontaneous processes with large entropy increases tend to be irreversible.
Safety note: While explosions perfectly demonstrate entropy principles, remember that firecrackers are dangerous and should only be handled by professionals!

Access to all documents
Improve your grades
Join milions of students
Temperature is a measurement of how hot or cold something is on a defined scale. But in chemistry, it's much more than just a comfort reading—it's a crucial factor in determining reaction spontaneity.
Temperature directly affects the entropy term in the Gibbs Free Energy equation. As temperature increases, the impact of entropy (ΔS) becomes more significant in determining whether a reaction will occur spontaneously.
When using the Gibbs equation , temperature must be in Kelvin (K), not Celsius or Fahrenheit. Remember that 0°C = 273.15 K to make accurate calculations.
Interesting fact: Some reactions that aren't spontaneous at room temperature become spontaneous at higher temperatures because the TΔS term grows large enough to overcome a positive ΔH!

Access to all documents
Improve your grades
Join milions of students
Gibbs Free Energy is the key thermodynamic concept that predicts whether chemical reactions will happen spontaneously. It combines both enthalpy and entropy effects into a single value.
When Gibbs Free Energy (ΔG) is negative, a reaction will occur on its own. When ΔG is positive, the reaction needs energy input to proceed. This simple rule helps chemists predict reaction behavior under specific conditions.
The beauty of Gibbs Free Energy is how it balances competing factors - the tendency to minimize energy (enthalpy) and maximize disorder (entropy). This balance explains why some endothermic reactions can still happen spontaneously!
Pro tip: The sign of ΔG is your quick indicator - negative means "go" and positive means "no" for spontaneous reactions.

Access to all documents
Improve your grades
Join milions of students
Gibbs Free Energy measures the maximum reversible work a system can perform at constant temperature and pressure. It's like a chemical reaction's "budget" for doing useful work.
When a reaction has negative ΔG, it releases free energy that can be harnessed to do work. This explains why exergonic reactions can power processes in both laboratory settings and living organisms.
The remarkable thing about Gibbs Free Energy is that it combines two competing natural tendencies - minimizing energy and maximizing disorder - into a single predictive value that tells us what will happen in the real world.
Key insight: Think of Gibbs Free Energy as nature's way of determining which direction processes will flow spontaneously, just like water flows downhill.

Access to all documents
Improve your grades
Join milions of students
Josiah Willard Gibbs developed what we now call Gibbs Free Energy in the 1870s. He initially termed it "available energy" in a system, highlighting its practical significance.
Gibbs was remarkably ahead of his time. His work on thermodynamics laid the foundation for modern chemical engineering, physical chemistry, and even quantum physics, though it wasn't widely recognized during his lifetime.
The concept of "available energy" perfectly captures the essence of Gibbs Free Energy - it's the energy in a system that's actually available to do useful work, not just the total energy present.
Historical note: Gibbs was the first American to earn a Ph.D. in engineering, and his contributions to science were so significant that Einstein called him "the greatest mind in American history."

Access to all documents
Improve your grades
Join milions of students
The Gibbs Free Energy equation is expressed as: ΔG° = ΔH° - TΔS°. This elegantly combines heat energy (enthalpy), disorder (entropy), and temperature into one powerful predictive tool.
Each component plays a crucial role: ΔH° represents the heat released or absorbed, ΔS° tracks changes in randomness, and T (temperature in Kelvin) determines how much weight entropy carries in the equation.
The beauty of this equation is its predictive power - it tells you immediately if a reaction will happen spontaneously (when ΔG is negative) or requires energy input (when ΔG is positive).
Visualization tip: Picture the equation as a balance scale with ΔH on one side and TΔS on the other. Whichever side weighs more determines the sign of ΔG!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
4
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
Explore the key concepts of energy transfer by heating, including black body radiation, thermal conductivity, and insulation techniques. This summary covers essential terms and principles related to conduction, infrared radiation, and effective heating strategies for buildings. Ideal for students studying thermal energy transfers.
Classification and Properties of fluid
Explore the principles of thermal energy transfer, including specific heat capacity, latent heat, and the mechanisms of conduction, convection, and radiation. This summary covers key concepts such as energy change calculations, the behavior of water as a coolant, and effective insulation methods. Ideal for students preparing for exams in physics or related subjects.
Explore the fundamental concepts of nuclear fusion and fission in this detailed summary. Understand the processes involved, energy production, and the differences between these two nuclear reactions. Key topics include the proton-proton cycle, fissionable isotopes, chain reactions, and energy outputs. Ideal for physics students seeking to grasp the principles of nuclear energy.
Explore the key concepts of nuclear fission and fusion, including their advantages, disadvantages, and the processes involved in energy production. This summary covers critical aspects such as chain reactions, safety concerns, and environmental impacts, making it essential for understanding nuclear power dynamics.
Explore the essential concepts of matter, including specific heat capacity, temperature, and density. This summary covers the behavior of solids, liquids, and gases, their phase changes, and the relationship between temperature and pressure. Ideal for students seeking a comprehensive understanding of physical properties in thermodynamics.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user