Periodic Trends
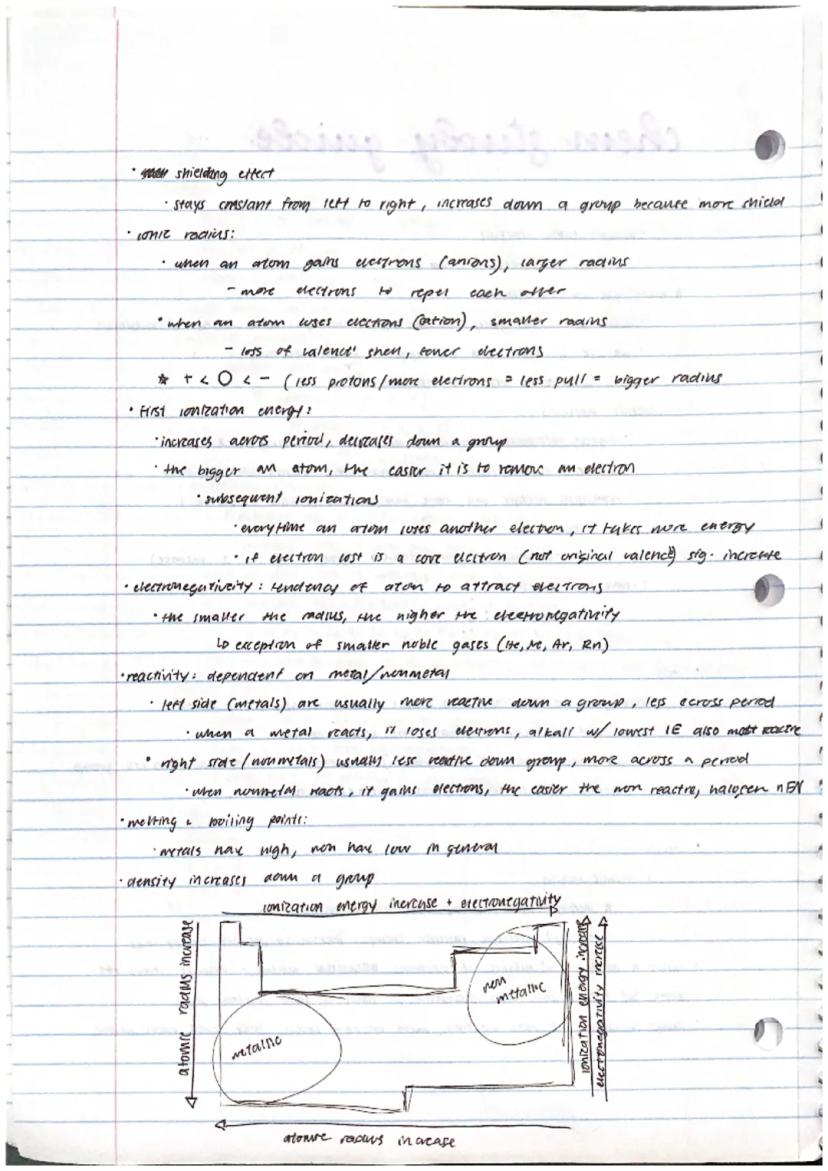
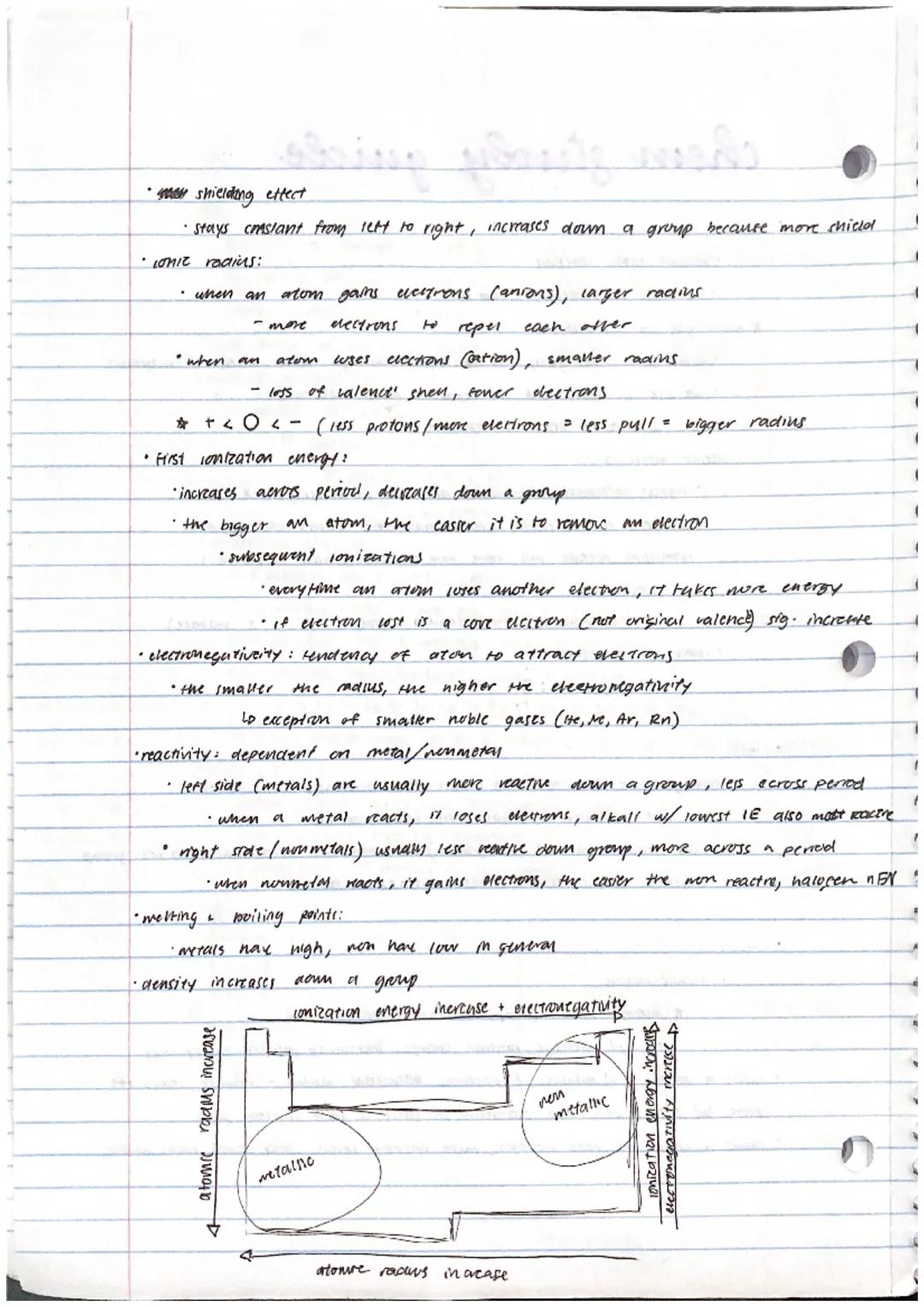
Atomic radius increases down a group but decreases across a period. Why? Down a group, more energy levels mean electrons are further from the nucleus. Across a period, the increasing number of protons pulls electrons tighter despite having the same energy level.
When atoms form ions, their size changes. Anions (negative ions) are larger than their neutral atoms because extra electrons increase repulsion. Cations (positive ions) are smaller than their atoms because they've lost electrons, reducing repulsion.
Ionization energy (energy needed to remove an electron) increases across periods and decreases down groups. Smaller atoms hold their electrons more tightly! Each subsequent electron removal requires more energy, with a significant jump when removing core non−valence electrons.
Electronegativity measures an atom's ability to attract shared electrons. It follows the same pattern as ionization energy—increasing across periods and decreasing down groups. This helps explain reactivity patterns: metals (which lose electrons) become more reactive down groups, while nonmetals (which gain electrons) become more reactive across periods.
Exam tip: Remember these trends by thinking about "nuclear pull" versus "distance." The stronger the nuclear pull and the shorter the distance between the nucleus and outer electrons, the smaller the atom, the higher the ionization energy, and the greater the electronegativity.