Thermodynamics rules our world - from engines that power cars... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Knowunity AI
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
180
•
Feb 18, 2026
•
Elizabeth Gabante
@elizabethgabante_mpdt
Thermodynamics rules our world - from engines that power cars... Show more
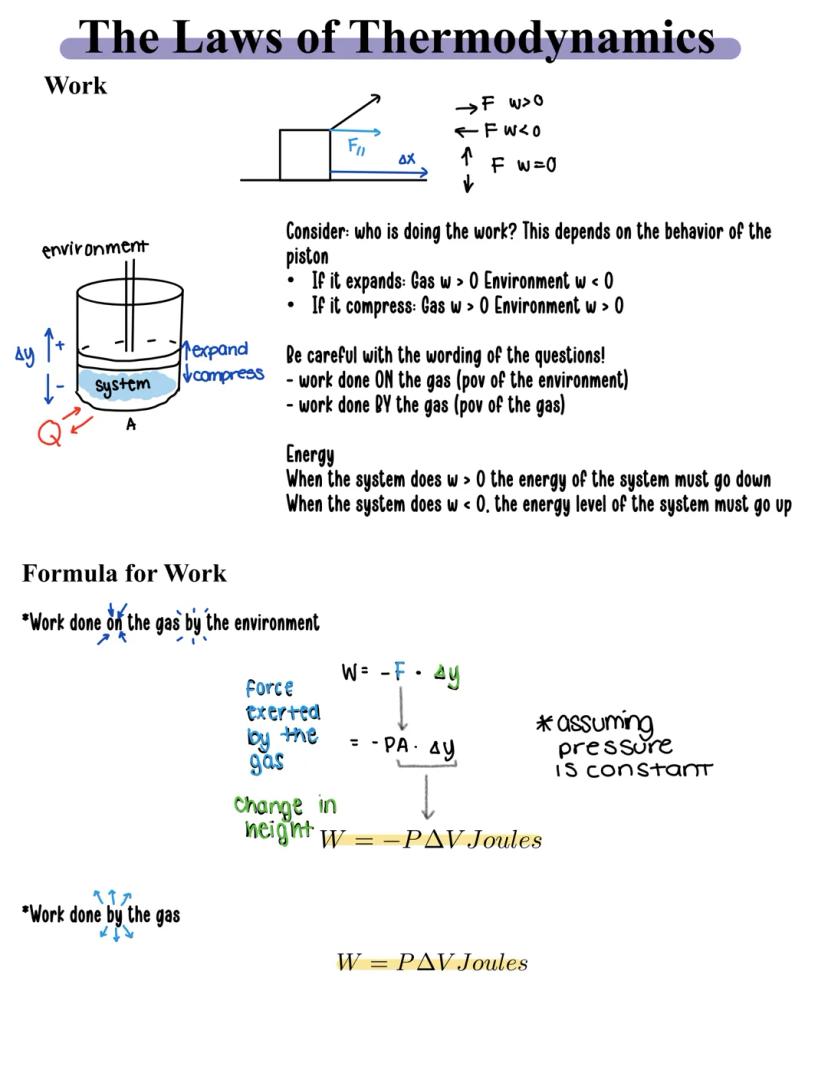





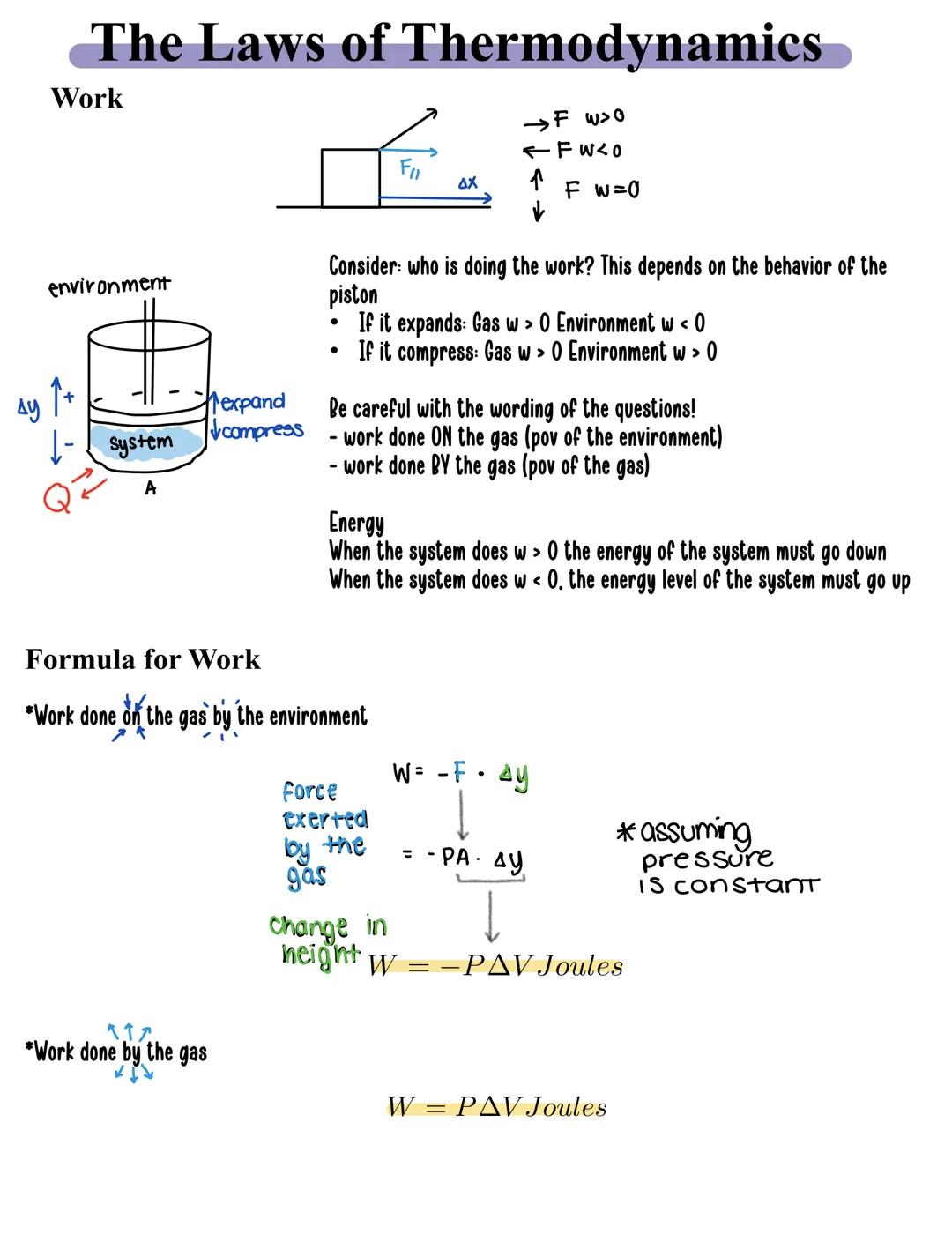
When gases expand or compress, they're doing work. Think about a piston in an engine - as gas pushes it outward, that's work being done by the gas. The direction matters:
The formula for work is straightforward: W = PΔV, where P is pressure and ΔV is the change in volume. But there's an important perspective shift to remember. When calculating:
Energy flows according to who's doing the work. When a system does positive work, its energy decreases. When work is done on the system, its energy increases.
Remember this! Always identify whose perspective you're using - the gas or the environment. This single detail determines whether your work value is positive or negative.

The area under a pressure-volume (PV) graph equals work. This visual representation makes it easy to understand energy transfer:
Work is path dependent, meaning how you get from state A to state B matters - not just the endpoints.
The First Law of Thermodynamics connects everything: ΔU = Q + W
This powerful equation is basically conservation of energy applied to thermodynamics. For example, if a gas absorbs 5000J of heat while doing 2000J of work, its internal energy increases by 3000J.
When solving problems with rigid containers, remember that W = 0 since the volume can't change. This simplifies the first law to ΔU = Q, meaning all heat added goes directly into increasing internal energy.
Pro tip: PV graphs are always from the environment's perspective. When tracing a path on the graph, think: right = expansion = negative work on system; left = compression = positive work on system.

Different gases store energy in different ways. Think of it like having various types of luggage - some have more compartments than others:
For monoatomic gases (like helium):
For diatomic gases (like oxygen):
Diatomic gases have higher heat capacity because they can store energy not just in the motion of atoms, but also in the bonds between them.
To solve thermodynamic problems systematically:
Remember this conversion: When working with thermodynamics, 1 liter = 0.001 m³. Converting units correctly is essential for accurate calculations.
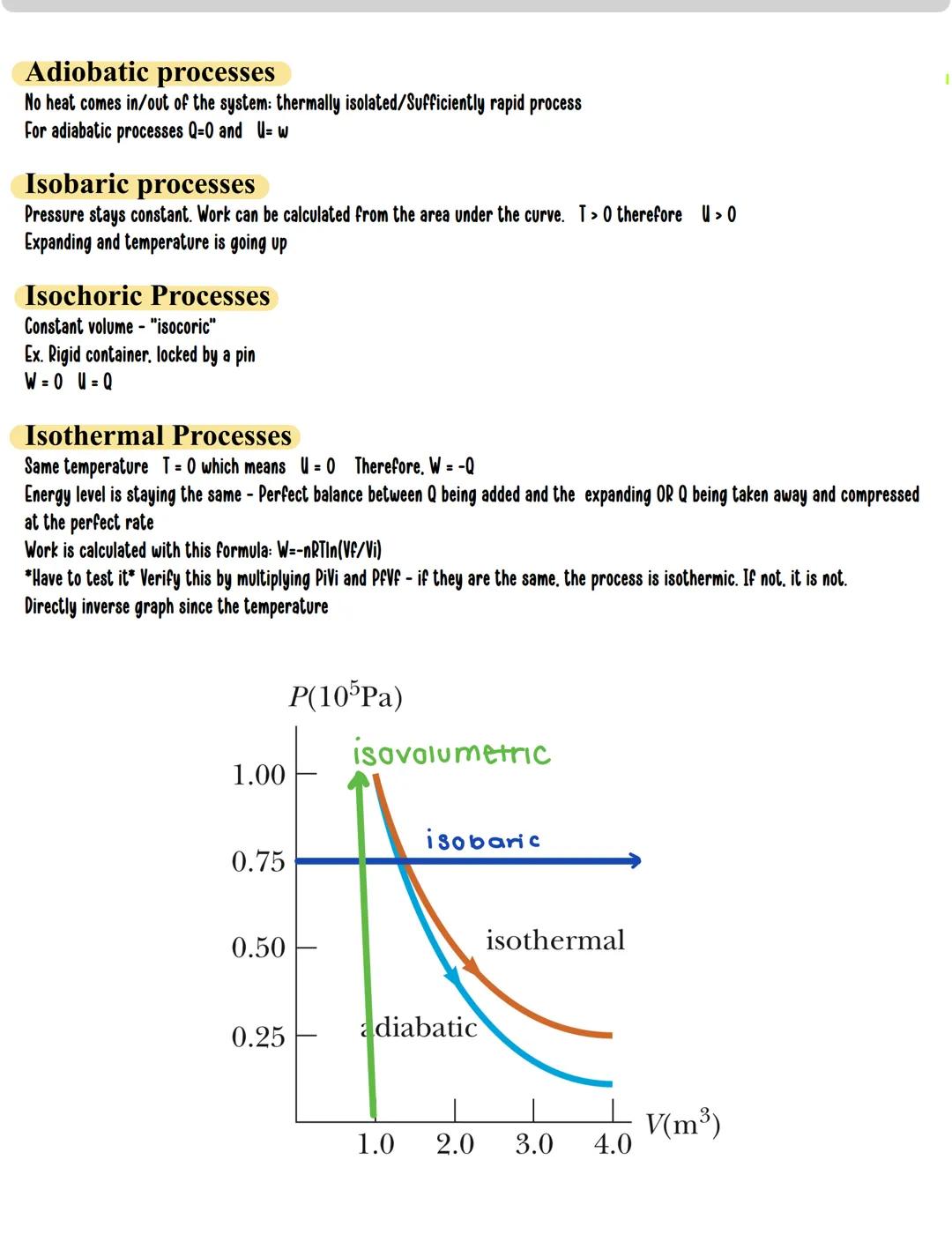
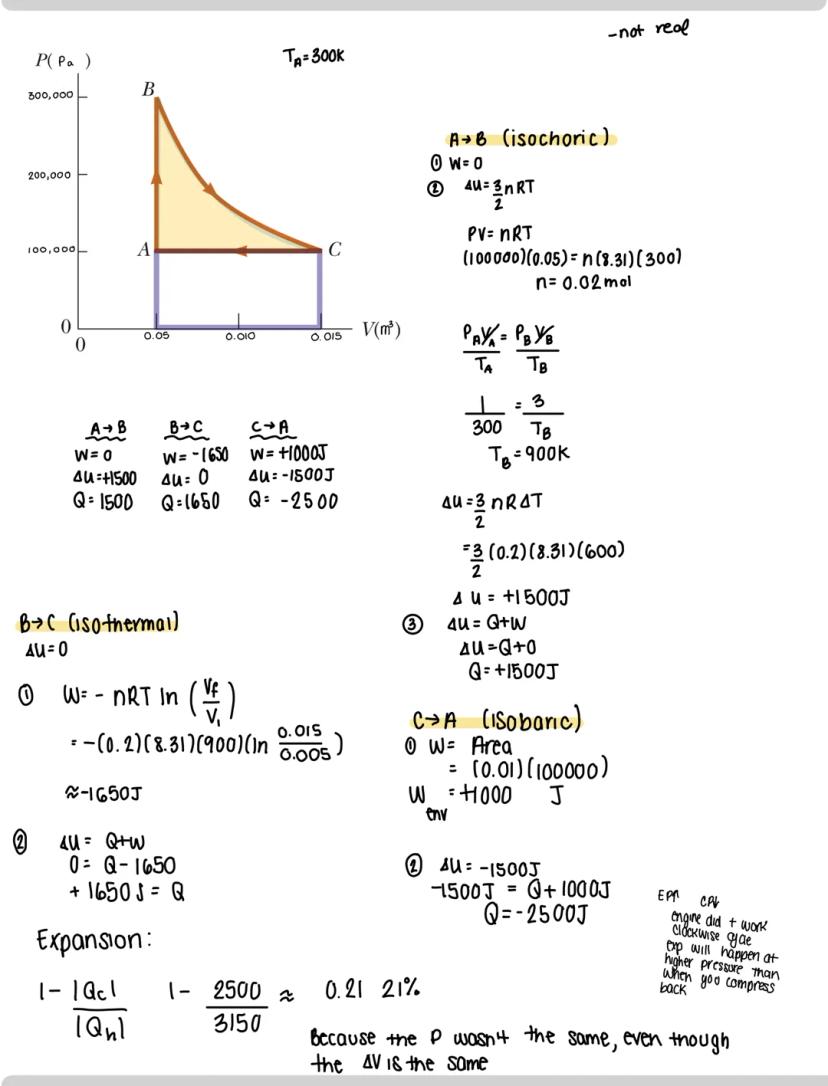
Different thermodynamic processes create distinctive PV curves and energy behaviors:
Adiabatic processes: No heat enters or leaves the system . This happens in thermally isolated systems or during extremely rapid changes. The relationship simplifies to ΔU = W.
Isobaric processes: Pressure remains constant. Work is easily calculated as W = PΔV. Since temperature typically changes, internal energy also changes (ΔU ≠ 0).
Isochoric processes: Volume remains constant (also called "isovolumetric"). Since there's no volume change, no work is done , which simplifies the first law to ΔU = Q.
Isothermal processes: Temperature stays constant , meaning internal energy doesn't change . This creates the relationship W = -Q - a perfect balance between heat and work. For isothermal processes, work is calculated using W = -nRTln.
Quick check for isothermal processes: Multiply PiVi and PfVf - if they're equal, the process is isothermal .

In isothermal expansions and compressions, temperature remains constant while volume and pressure change. The formula for work becomes: W = nRTln
This formula gives us insights about work direction:
Cyclic processes are fascinating because they return to their starting point. The work done in a cyclic process equals the area enclosed by the curve on a PV diagram. The direction of the cycle matters:
When solving complex thermodynamic problems, break them into steps:
Visualization tip: Draw the PV diagram whenever possible. It helps identify the processes and makes calculating work much more intuitive through the "area under the curve" concept.
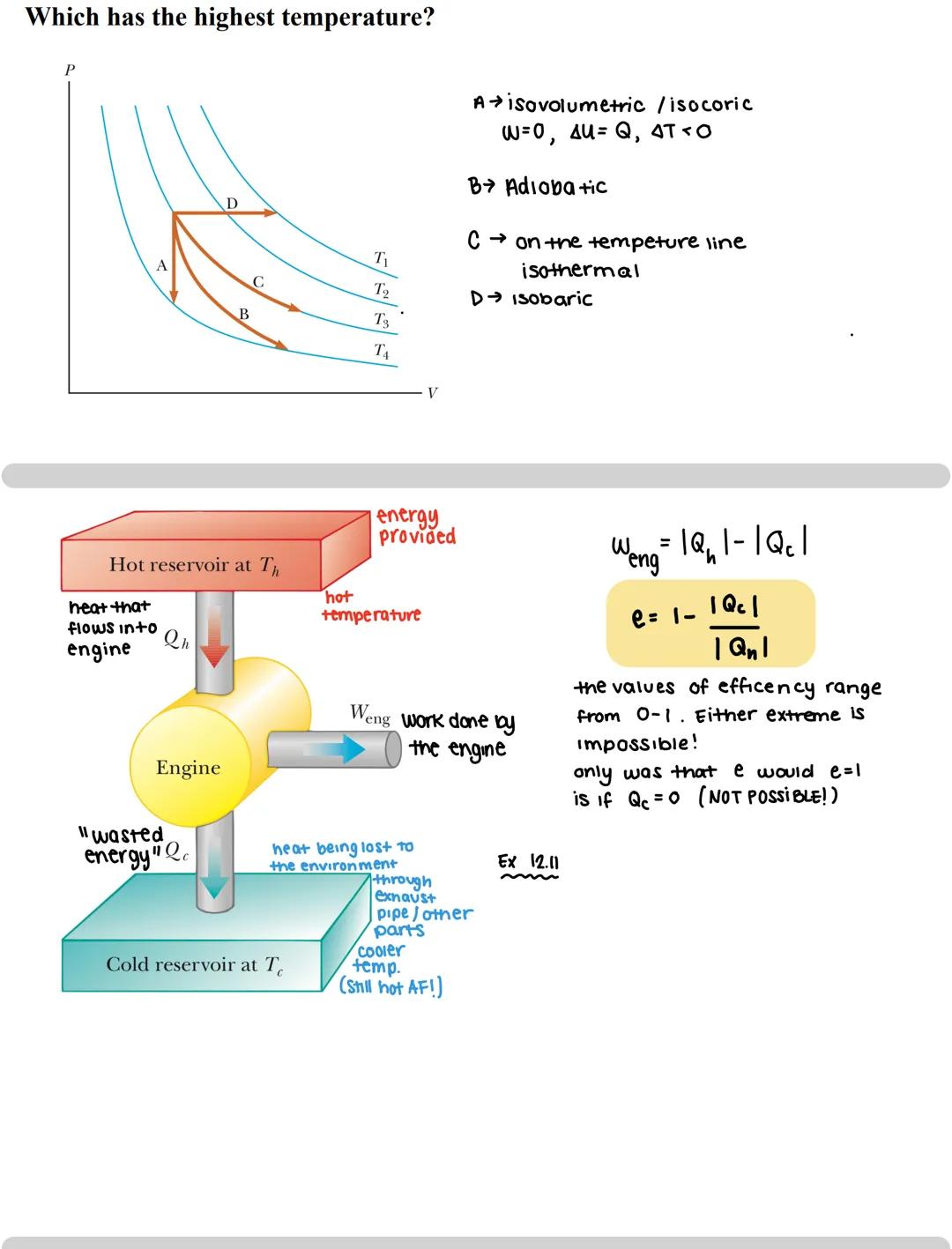
Heat engines convert thermal energy into mechanical work by moving heat from a hot reservoir to a cold one. Think of your car engine - it burns fuel (hot reservoir), does work (moves your car), and expels waste heat through the exhaust (cold reservoir).
The key formula for an engine is: Weng = |Qh| - |Qc| Where:
Efficiency is the ratio of useful work to the heat input: e = Weng/|Qh| = 1 - |Qc|/|Qh|
Perfect efficiency would require Qc = 0, meaning no heat rejected to the cold reservoir. However, this is physically impossible according to the Second Law of Thermodynamics - some energy must always be "wasted."
When analyzing temperature in PV diagrams, hyperbolas (curves) represent isotherms - lines of constant temperature. The further from the origin, the higher the temperature. For example, in a PV diagram with points A, B, C, and D, you can determine which point has the highest temperature by seeing which lies on the outermost curve.
Real-world connection: This is why your car's engine feels hot and why it needs a cooling system - physics dictates that engines cannot convert all their heat into work!
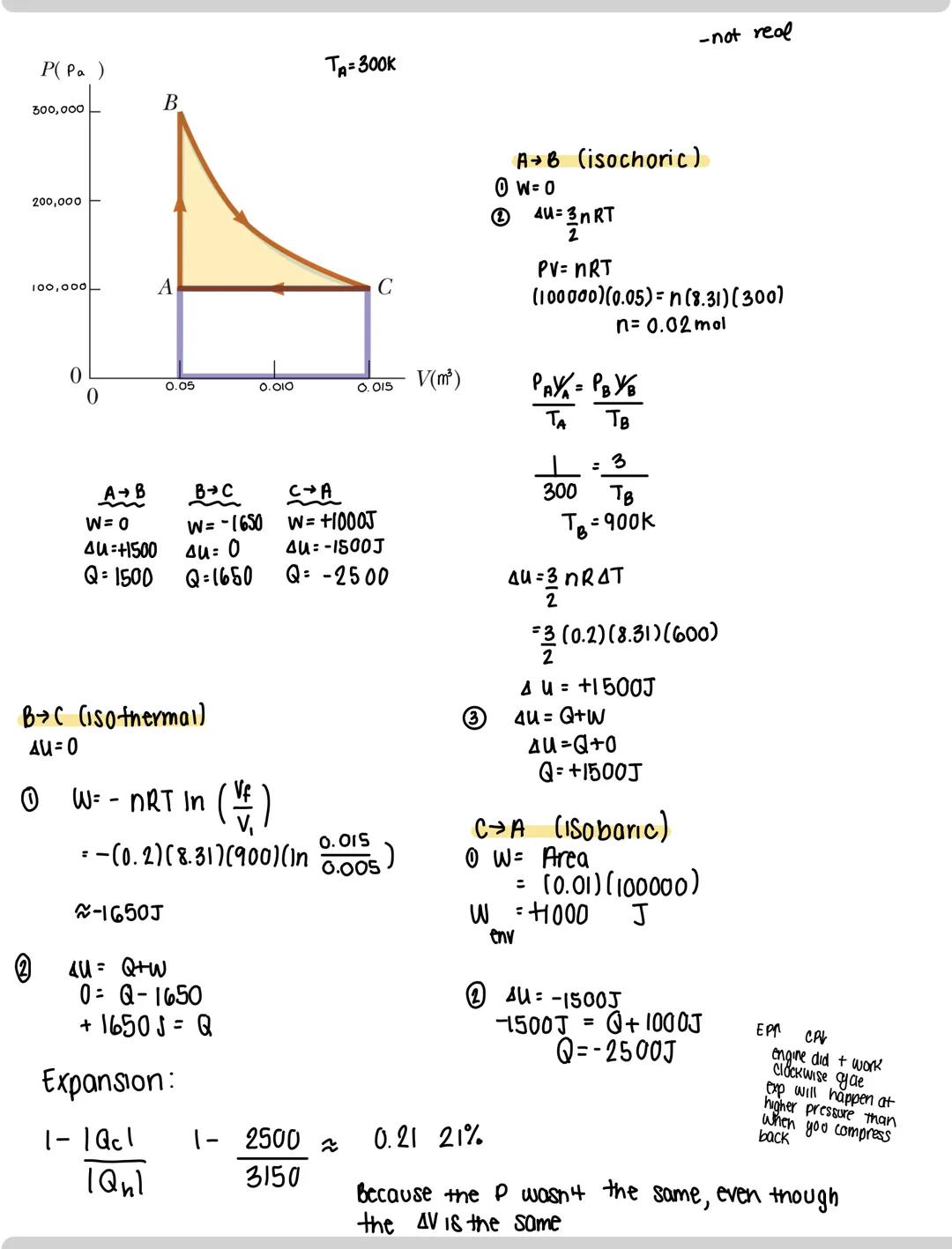
Cyclic processes are powerful tools for understanding thermodynamic systems. In a cycle, the system returns to its initial state after performing a series of processes. Let's analyze a typical cycle:
When working with a cycle (A→B→C→A), break it into segments:
For each segment, calculate:
The direction of the cycle determines overall behavior:
The key insight: expansion typically happens at higher pressure than compression, which is why engines can produce net work.
Physics insight: Work in a cycle depends on path, not just endpoints. Two cycles between the same states can have completely different work values depending on the path taken.
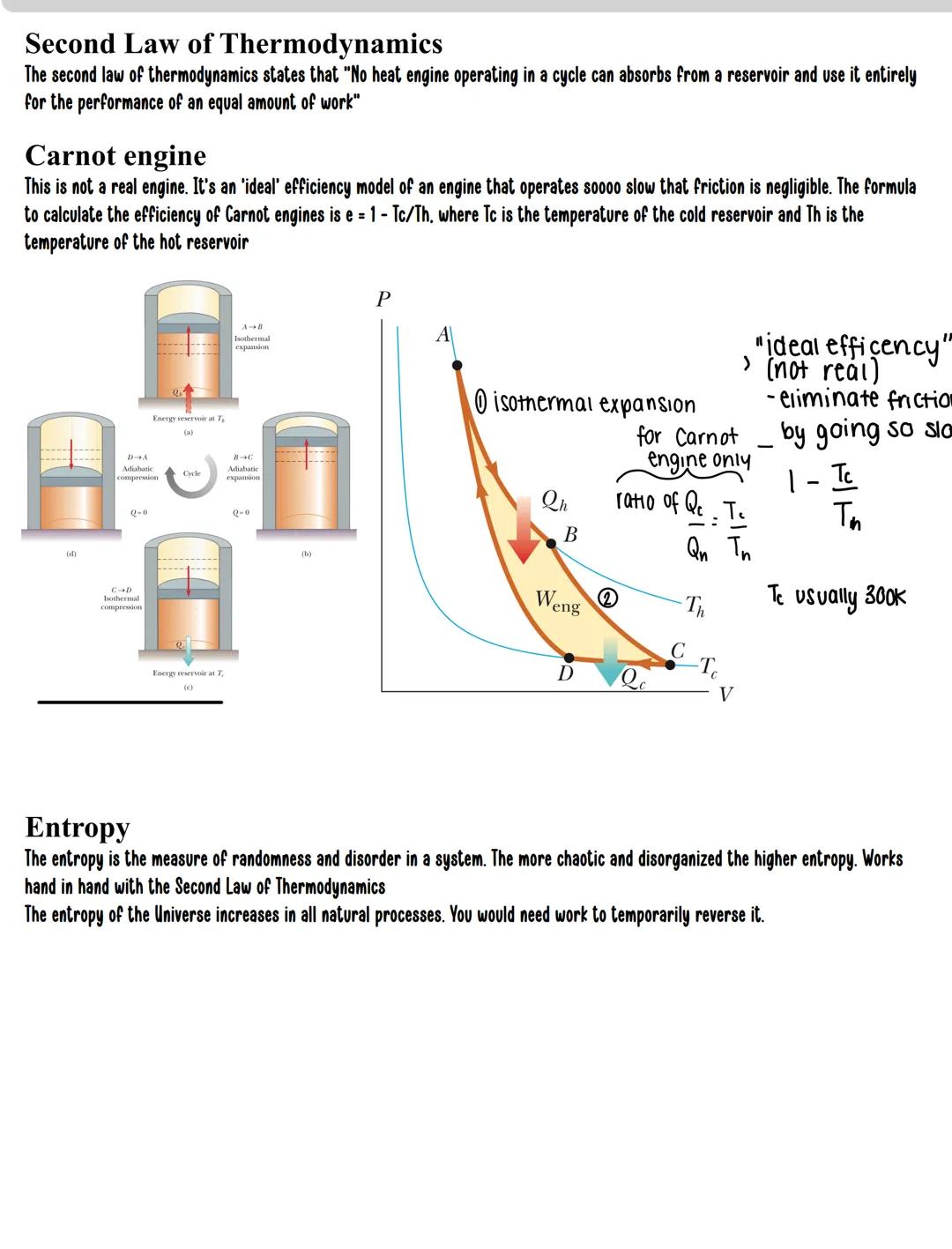
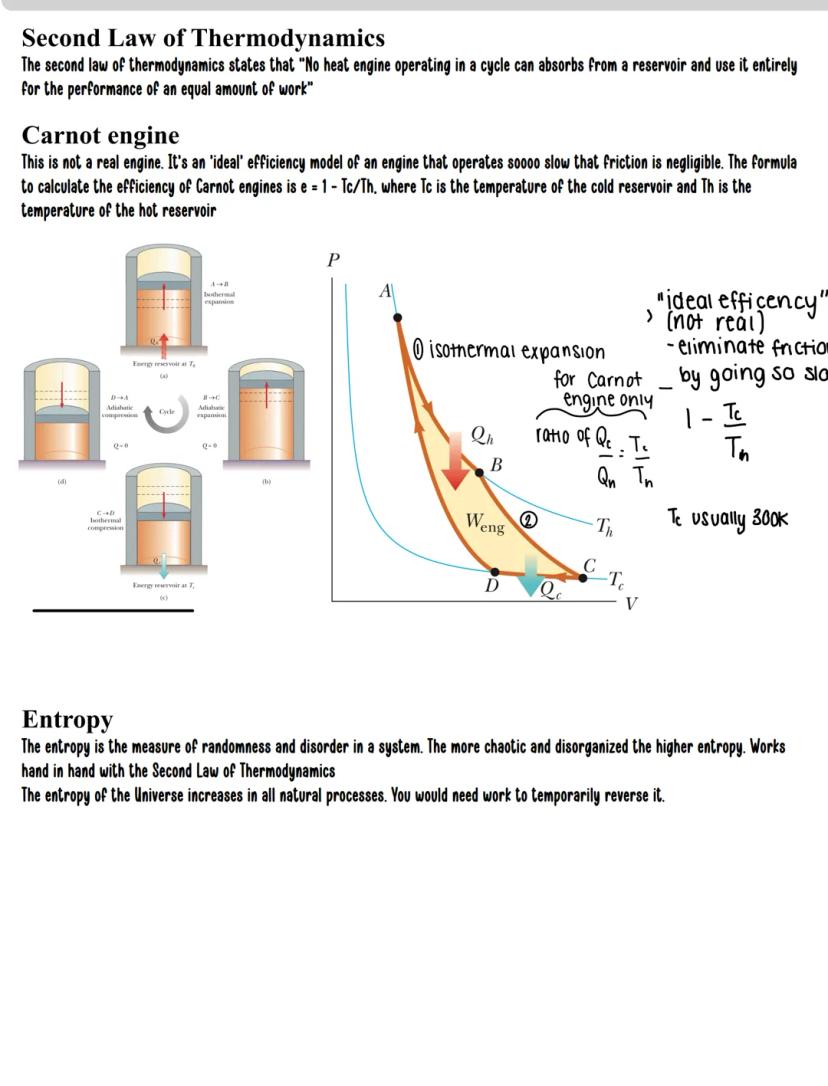
The Second Law of Thermodynamics deals with the direction of natural processes. It states that no heat engine operating in a cycle can convert all heat absorbed into work - some heat must always be rejected to a colder reservoir.
The Carnot engine represents the theoretical maximum efficiency possible. It operates through four stages:
The Carnot efficiency formula is elegantly simple: e = 1 - Tc/Th
Where:
This maximum possible efficiency depends solely on temperature ratio. For example, if Th = 600K and Tc = 300K, the maximum efficiency is 50%, regardless of engine design.
Entropy is the measure of disorder in a system. The Second Law can be restated: the total entropy of the universe always increases in natural processes. Creating order in one place requires creating more disorder elsewhere.
Real-world application: This is why your room naturally becomes messy and requires work to clean up! Increasing organization in one part of the universe requires increasing disorder elsewhere.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Elizabeth Gabante
@elizabethgabante_mpdt
Thermodynamics rules our world - from engines that power cars to chemical reactions in your body. These powerful laws describe how energy moves, transforms, and ultimately dictates what's physically possible in our universe. Let's break down the key concepts that... Show more

Access to all documents
Improve your grades
Join milions of students
When gases expand or compress, they're doing work. Think about a piston in an engine - as gas pushes it outward, that's work being done by the gas. The direction matters:
The formula for work is straightforward: W = PΔV, where P is pressure and ΔV is the change in volume. But there's an important perspective shift to remember. When calculating:
Energy flows according to who's doing the work. When a system does positive work, its energy decreases. When work is done on the system, its energy increases.
Remember this! Always identify whose perspective you're using - the gas or the environment. This single detail determines whether your work value is positive or negative.

Access to all documents
Improve your grades
Join milions of students
The area under a pressure-volume (PV) graph equals work. This visual representation makes it easy to understand energy transfer:
Work is path dependent, meaning how you get from state A to state B matters - not just the endpoints.
The First Law of Thermodynamics connects everything: ΔU = Q + W
This powerful equation is basically conservation of energy applied to thermodynamics. For example, if a gas absorbs 5000J of heat while doing 2000J of work, its internal energy increases by 3000J.
When solving problems with rigid containers, remember that W = 0 since the volume can't change. This simplifies the first law to ΔU = Q, meaning all heat added goes directly into increasing internal energy.
Pro tip: PV graphs are always from the environment's perspective. When tracing a path on the graph, think: right = expansion = negative work on system; left = compression = positive work on system.

Access to all documents
Improve your grades
Join milions of students
Different gases store energy in different ways. Think of it like having various types of luggage - some have more compartments than others:
For monoatomic gases (like helium):
For diatomic gases (like oxygen):
Diatomic gases have higher heat capacity because they can store energy not just in the motion of atoms, but also in the bonds between them.
To solve thermodynamic problems systematically:
Remember this conversion: When working with thermodynamics, 1 liter = 0.001 m³. Converting units correctly is essential for accurate calculations.

Access to all documents
Improve your grades
Join milions of students
Different thermodynamic processes create distinctive PV curves and energy behaviors:
Adiabatic processes: No heat enters or leaves the system . This happens in thermally isolated systems or during extremely rapid changes. The relationship simplifies to ΔU = W.
Isobaric processes: Pressure remains constant. Work is easily calculated as W = PΔV. Since temperature typically changes, internal energy also changes (ΔU ≠ 0).
Isochoric processes: Volume remains constant (also called "isovolumetric"). Since there's no volume change, no work is done , which simplifies the first law to ΔU = Q.
Isothermal processes: Temperature stays constant , meaning internal energy doesn't change . This creates the relationship W = -Q - a perfect balance between heat and work. For isothermal processes, work is calculated using W = -nRTln.
Quick check for isothermal processes: Multiply PiVi and PfVf - if they're equal, the process is isothermal .

Access to all documents
Improve your grades
Join milions of students
In isothermal expansions and compressions, temperature remains constant while volume and pressure change. The formula for work becomes: W = nRTln
This formula gives us insights about work direction:
Cyclic processes are fascinating because they return to their starting point. The work done in a cyclic process equals the area enclosed by the curve on a PV diagram. The direction of the cycle matters:
When solving complex thermodynamic problems, break them into steps:
Visualization tip: Draw the PV diagram whenever possible. It helps identify the processes and makes calculating work much more intuitive through the "area under the curve" concept.

Access to all documents
Improve your grades
Join milions of students
Heat engines convert thermal energy into mechanical work by moving heat from a hot reservoir to a cold one. Think of your car engine - it burns fuel (hot reservoir), does work (moves your car), and expels waste heat through the exhaust (cold reservoir).
The key formula for an engine is: Weng = |Qh| - |Qc| Where:
Efficiency is the ratio of useful work to the heat input: e = Weng/|Qh| = 1 - |Qc|/|Qh|
Perfect efficiency would require Qc = 0, meaning no heat rejected to the cold reservoir. However, this is physically impossible according to the Second Law of Thermodynamics - some energy must always be "wasted."
When analyzing temperature in PV diagrams, hyperbolas (curves) represent isotherms - lines of constant temperature. The further from the origin, the higher the temperature. For example, in a PV diagram with points A, B, C, and D, you can determine which point has the highest temperature by seeing which lies on the outermost curve.
Real-world connection: This is why your car's engine feels hot and why it needs a cooling system - physics dictates that engines cannot convert all their heat into work!

Access to all documents
Improve your grades
Join milions of students
Cyclic processes are powerful tools for understanding thermodynamic systems. In a cycle, the system returns to its initial state after performing a series of processes. Let's analyze a typical cycle:
When working with a cycle (A→B→C→A), break it into segments:
For each segment, calculate:
The direction of the cycle determines overall behavior:
The key insight: expansion typically happens at higher pressure than compression, which is why engines can produce net work.
Physics insight: Work in a cycle depends on path, not just endpoints. Two cycles between the same states can have completely different work values depending on the path taken.

Access to all documents
Improve your grades
Join milions of students
The Second Law of Thermodynamics deals with the direction of natural processes. It states that no heat engine operating in a cycle can convert all heat absorbed into work - some heat must always be rejected to a colder reservoir.
The Carnot engine represents the theoretical maximum efficiency possible. It operates through four stages:
The Carnot efficiency formula is elegantly simple: e = 1 - Tc/Th
Where:
This maximum possible efficiency depends solely on temperature ratio. For example, if Th = 600K and Tc = 300K, the maximum efficiency is 50%, regardless of engine design.
Entropy is the measure of disorder in a system. The Second Law can be restated: the total entropy of the universe always increases in natural processes. Creating order in one place requires creating more disorder elsewhere.
Real-world application: This is why your room naturally becomes messy and requires work to clean up! Increasing organization in one part of the universe requires increasing disorder elsewhere.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
5
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
This includes the basic concepts in physics like mass, weight, pressure, density, energy and etc., and how to solve them.
An honors intro to physics work and energy study guide
This presentation offers an explanation of simple machines and how to solve for mechanical advantage, ideal mechanical advantage, and efficiency of each machine. It also provides definitions and examples.
This helps in remembering important formulas in physics.
The electric circuit notes are good for an intro to physics class.
Explore the essential features of the Periodic Table, including atomic structure, groups, and trends. This summary highlights key elements, their atomic numbers, and properties, making it a valuable resource for understanding chemical elements and their classifications. Ideal for students studying chemistry.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user