The periodic table is a powerful organizational tool that groups... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
Subjects
Triangle Congruence and Similarity Theorems
Triangle Properties and Classification
Linear Equations and Graphs
Geometric Angle Relationships
Trigonometric Functions and Identities
Equation Solving Techniques
Circle Geometry Fundamentals
Division Operations and Methods
Basic Differentiation Rules
Exponent and Logarithm Properties
Show all topics
Human Organ Systems
Reproductive Cell Cycles
Biological Sciences Subdisciplines
Cellular Energy Metabolism
Autotrophic Energy Processes
Inheritance Patterns and Principles
Biomolecular Structure and Organization
Cell Cycle and Division Mechanics
Cellular Organization and Development
Biological Structural Organization
Show all topics
Chemical Sciences and Applications
Atomic Structure and Composition
Molecular Electron Structure Representation
Atomic Electron Behavior
Matter Properties and Water
Mole Concept and Calculations
Gas Laws and Behavior
Periodic Table Organization
Chemical Thermodynamics Fundamentals
Chemical Bond Types and Properties
Show all topics
European Renaissance and Enlightenment
European Cultural Movements 800-1920
American Revolution Era 1763-1797
American Civil War 1861-1865
Global Imperial Systems
Mongol and Chinese Dynasties
U.S. Presidents and World Leaders
Historical Sources and Documentation
World Wars Era and Impact
World Religious Systems
Show all topics
Classic and Contemporary Novels
Literary Character Analysis
Rhetorical Theory and Practice
Classic Literary Narratives
Reading Analysis and Interpretation
Narrative Structure and Techniques
English Language Components
Influential English-Language Authors
Basic Sentence Structure
Narrative Voice and Perspective
Show all topics
21
•
Feb 1, 2026
•
Skylar
@kylaraffone_mlhy
The periodic table is a powerful organizational tool that groups... Show more
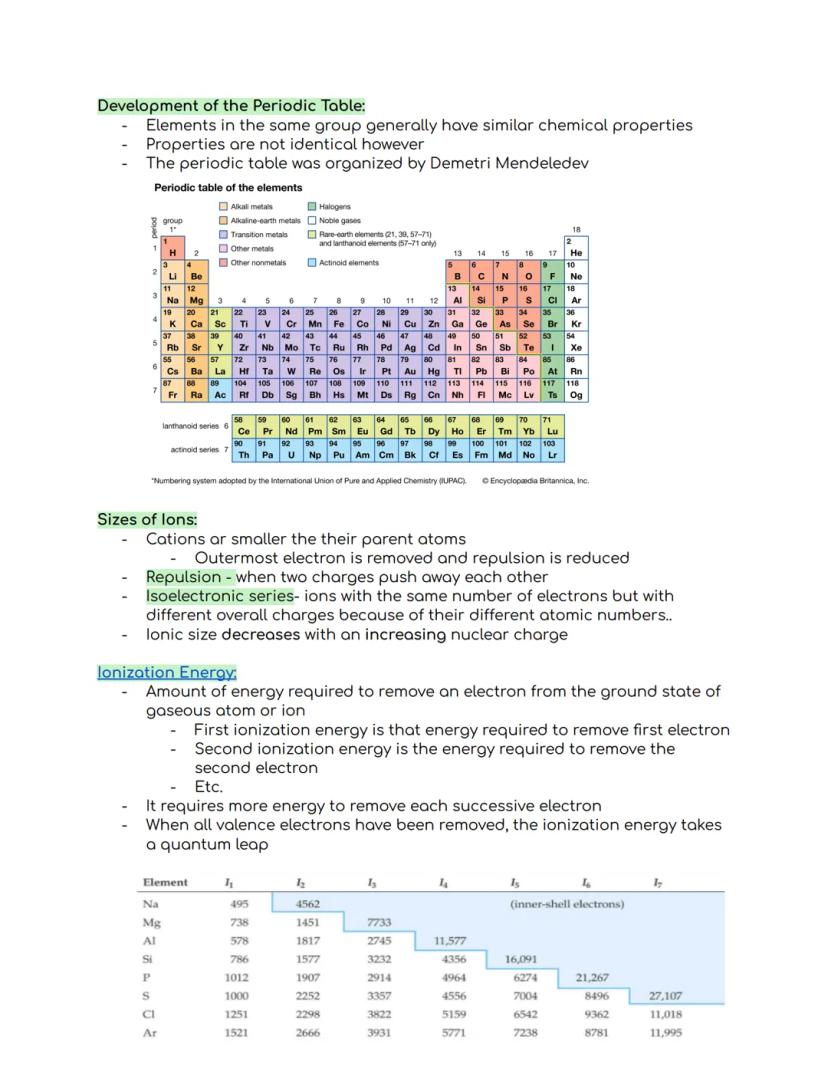

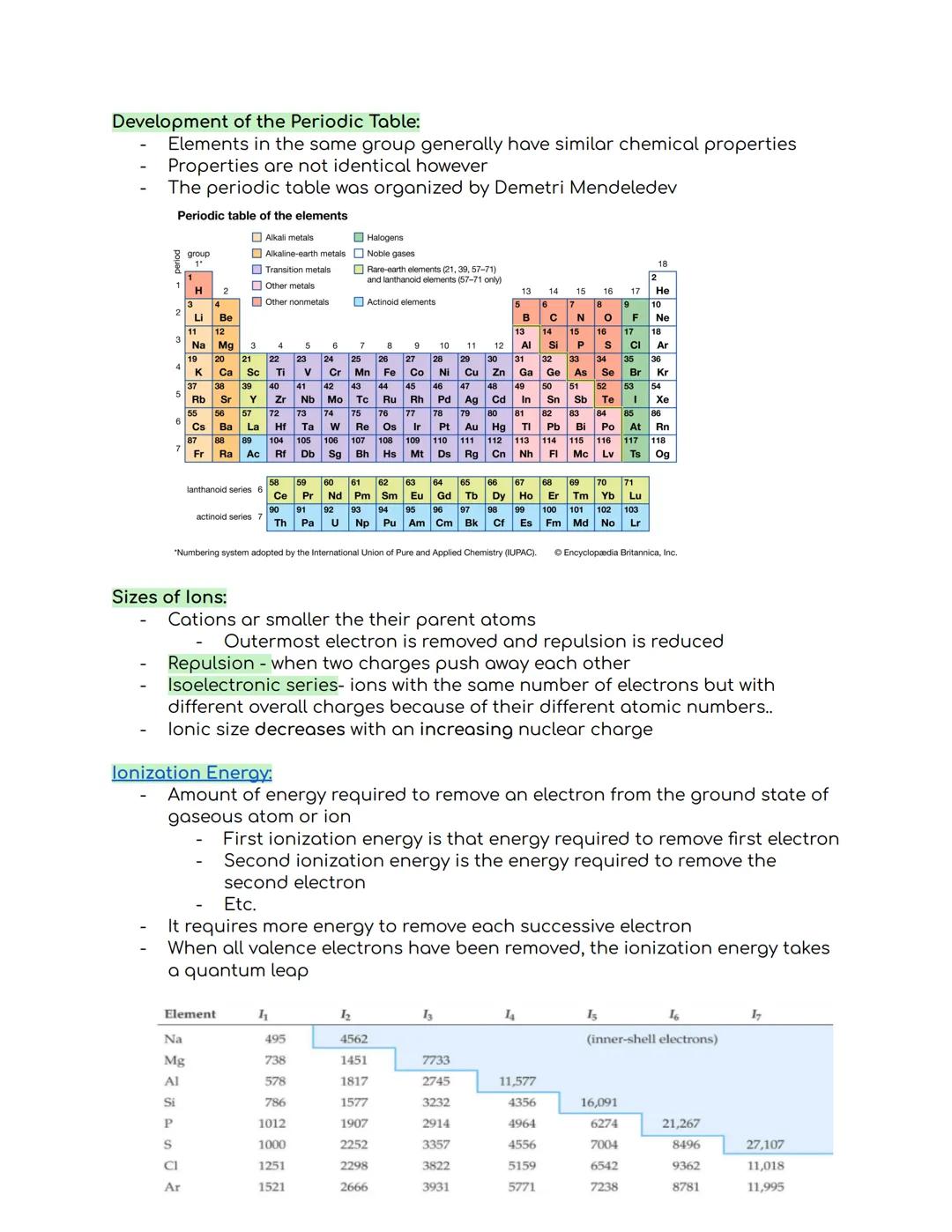
The periodic table organizes elements into groups (columns) and periods (rows), with elements in the same group sharing similar chemical properties. Dmitri Mendeleev created this organizational system, which has become essential for understanding chemistry.
When we look at ions, cations (positively charged ions) are smaller than their parent atoms because removing electrons reduces repulsion between remaining electrons. An isoelectronic series contains ions with identical electron counts but different charges due to their varying atomic numbers.
Ionization energy measures how much energy is needed to remove an electron from an atom. The first ionization energy removes the first electron, the second removes the next, and so on. Each successive electron requires more energy to remove, with a huge jump occurring after all valence electrons are gone.
💡 Think of ionization energy like pulling children away from a parent - the first child might leave easily, but each additional child becomes harder to separate, with a dramatic increase in difficulty when you reach the parent's favorite child (inner electrons)!
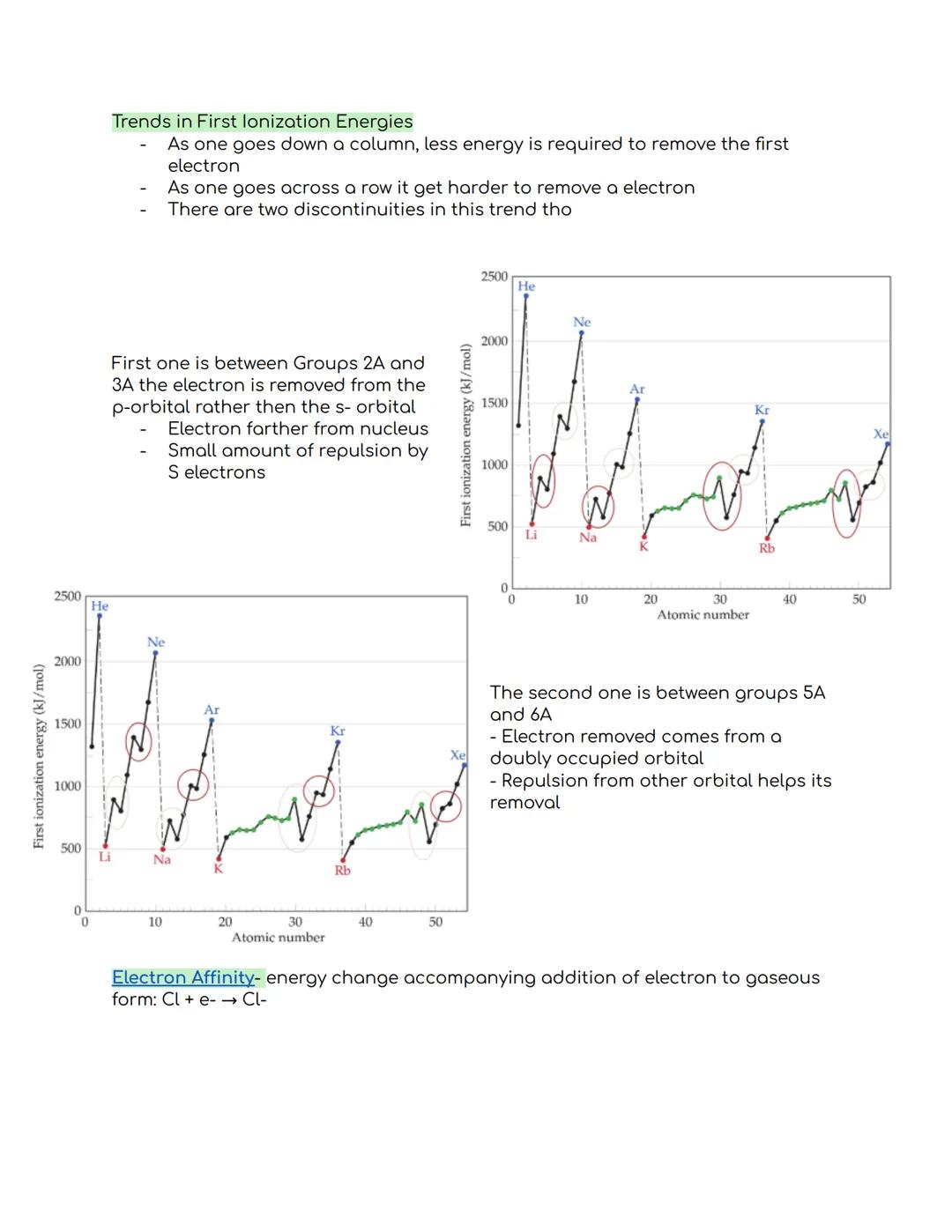
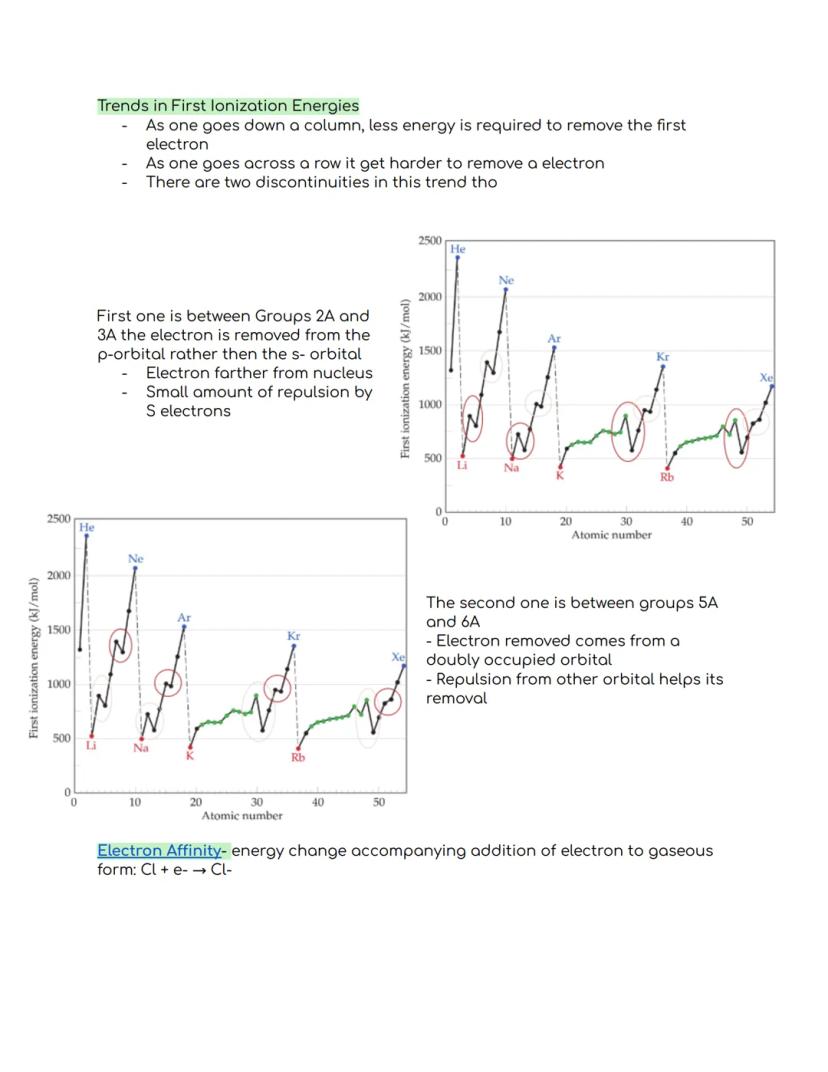
As you move down a group in the periodic table, first ionization energy decreases. This happens because valence electrons are farther from the nucleus in larger atoms, making them easier to remove. Conversely, moving across a period from left to right generally makes it harder to remove electrons.
There are two interesting exceptions to this trend. Between Groups 2A and 3A, ionization energy drops because the electron comes from a p-orbital rather than an s-orbital. The electron is farther from the nucleus and experiences some repulsion from s-electrons, making it easier to remove.
The second exception occurs between Groups 5A and 6A, where ionization energy decreases because the electron being removed comes from a doubly occupied orbital. The repulsion between electrons in the same orbital actually helps the electron leave.
Electron affinity measures the energy change when a neutral atom gains an electron. Some elements release energy (negative value) when gaining electrons, while others require energy (positive value).

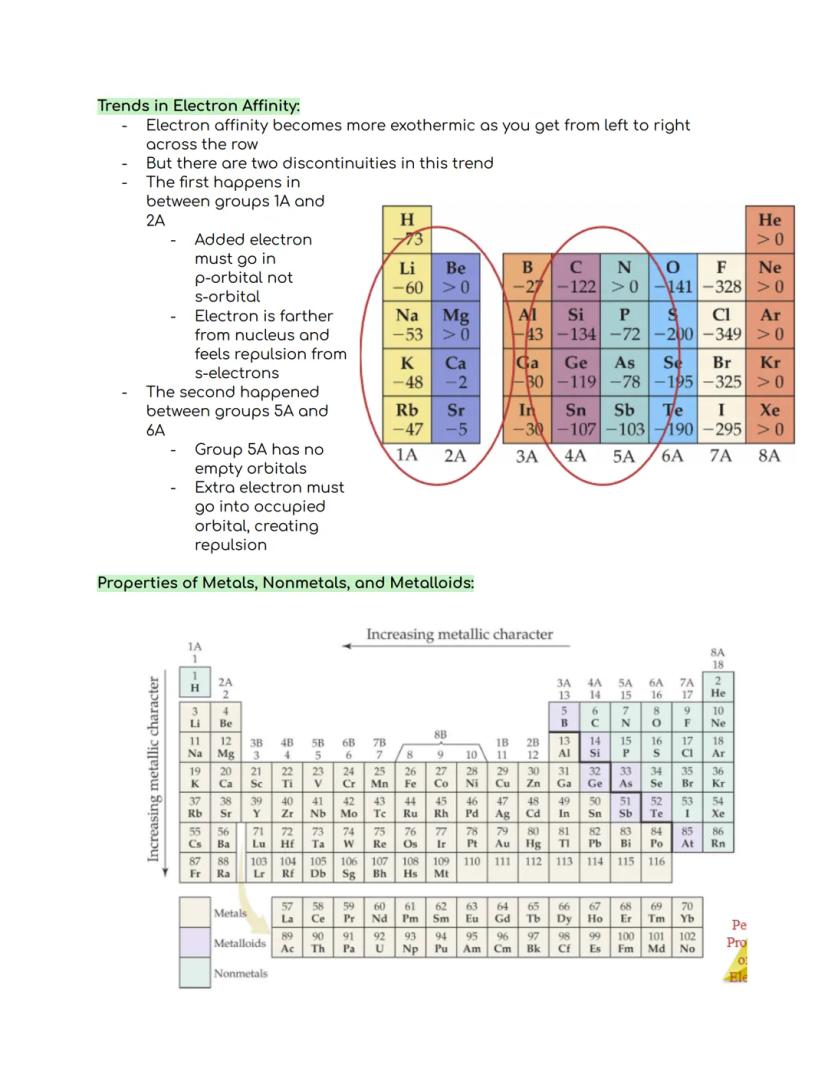
Electron affinity generally becomes more exothermic as you move from left to right across a period. This means elements on the right side of the table are more eager to gain electrons than those on the left.
Just like with ionization energy, there are two exceptions. Between Groups 1A and 2A, electron affinity becomes less favorable because the added electron must enter a p-orbital instead of an s-orbital. This puts the electron farther from the nucleus and creates repulsion with s-electrons.
The second exception happens between Groups 5A and 6A. Group 5A elements have no empty orbitals left, so an extra electron must squeeze into an already occupied orbital. This creates repulsion between electrons, making it less favorable.
🔍 Metallic character increases as you move down a group and decreases as you move across a period. This explains why elements in the bottom left of the table are the most metallic, while those in the top right are strongly nonmetallic.

Metals and nonmetals have distinctly different properties. Metals typically have a shiny luster, are good conductors of heat and electricity, and can be shaped (malleable and ductile). Their oxides form basic solutions, and they tend to form positive ions (cations) in solution.
Nonmetals lack metallic luster, are usually poor conductors, and solids are often brittle. Their oxides form acidic solutions, and they typically form negative ions (anions) in solution.
Alkali metals (Group 1A) are extremely reactive, soft metals with low melting points and densities. They react vigorously with water, producing hydrogen gas and heat. Each element produces a distinctive flame color, which is useful for identification.
Alkaline earth metals (Group 2A) are less reactive than alkali metals but still quite reactive. They have higher melting points and densities, with reactivity increasing down the group.
Halogens (Group 7A) have large negative electron affinities, making them excellent oxidizing agents. They readily form compounds with metals called metal halides. In contrast, noble gases (Group 8A) have high ionization energies and positive electron affinities, making them extremely unreactive.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Skylar
@kylaraffone_mlhy
The periodic table is a powerful organizational tool that groups elements based on their chemical properties. Created by Dmitri Mendeleev, it arranges elements in a way that reveals patterns in their behavior, helping us predict how they'll interact with each... Show more

Access to all documents
Improve your grades
Join milions of students
The periodic table organizes elements into groups (columns) and periods (rows), with elements in the same group sharing similar chemical properties. Dmitri Mendeleev created this organizational system, which has become essential for understanding chemistry.
When we look at ions, cations (positively charged ions) are smaller than their parent atoms because removing electrons reduces repulsion between remaining electrons. An isoelectronic series contains ions with identical electron counts but different charges due to their varying atomic numbers.
Ionization energy measures how much energy is needed to remove an electron from an atom. The first ionization energy removes the first electron, the second removes the next, and so on. Each successive electron requires more energy to remove, with a huge jump occurring after all valence electrons are gone.
💡 Think of ionization energy like pulling children away from a parent - the first child might leave easily, but each additional child becomes harder to separate, with a dramatic increase in difficulty when you reach the parent's favorite child (inner electrons)!

Access to all documents
Improve your grades
Join milions of students
As you move down a group in the periodic table, first ionization energy decreases. This happens because valence electrons are farther from the nucleus in larger atoms, making them easier to remove. Conversely, moving across a period from left to right generally makes it harder to remove electrons.
There are two interesting exceptions to this trend. Between Groups 2A and 3A, ionization energy drops because the electron comes from a p-orbital rather than an s-orbital. The electron is farther from the nucleus and experiences some repulsion from s-electrons, making it easier to remove.
The second exception occurs between Groups 5A and 6A, where ionization energy decreases because the electron being removed comes from a doubly occupied orbital. The repulsion between electrons in the same orbital actually helps the electron leave.
Electron affinity measures the energy change when a neutral atom gains an electron. Some elements release energy (negative value) when gaining electrons, while others require energy (positive value).

Access to all documents
Improve your grades
Join milions of students
Electron affinity generally becomes more exothermic as you move from left to right across a period. This means elements on the right side of the table are more eager to gain electrons than those on the left.
Just like with ionization energy, there are two exceptions. Between Groups 1A and 2A, electron affinity becomes less favorable because the added electron must enter a p-orbital instead of an s-orbital. This puts the electron farther from the nucleus and creates repulsion with s-electrons.
The second exception happens between Groups 5A and 6A. Group 5A elements have no empty orbitals left, so an extra electron must squeeze into an already occupied orbital. This creates repulsion between electrons, making it less favorable.
🔍 Metallic character increases as you move down a group and decreases as you move across a period. This explains why elements in the bottom left of the table are the most metallic, while those in the top right are strongly nonmetallic.

Access to all documents
Improve your grades
Join milions of students
Metals and nonmetals have distinctly different properties. Metals typically have a shiny luster, are good conductors of heat and electricity, and can be shaped (malleable and ductile). Their oxides form basic solutions, and they tend to form positive ions (cations) in solution.
Nonmetals lack metallic luster, are usually poor conductors, and solids are often brittle. Their oxides form acidic solutions, and they typically form negative ions (anions) in solution.
Alkali metals (Group 1A) are extremely reactive, soft metals with low melting points and densities. They react vigorously with water, producing hydrogen gas and heat. Each element produces a distinctive flame color, which is useful for identification.
Alkaline earth metals (Group 2A) are less reactive than alkali metals but still quite reactive. They have higher melting points and densities, with reactivity increasing down the group.
Halogens (Group 7A) have large negative electron affinities, making them excellent oxidizing agents. They readily form compounds with metals called metal halides. In contrast, noble gases (Group 8A) have high ionization energies and positive electron affinities, making them extremely unreactive.
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
0
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Practice Test ✓ Essay Outlines
Notes on the periodic table, atoms, elements, and more
Explore the fundamentals of atomic structure and the periodic table in this comprehensive summary. Key concepts include atomic composition, isotopes, ions, and the development of the periodic table by Mendeleev and Newlands. Ideal for students preparing for exams, this resource features clear explanations and a simplified mind map for better understanding.
Lists the 5 states of matter, how they can be defined when split, and introduces the parts of the symbols/labels on the Periodic Table
Handwritten notes based on the information in Unit 6: The Periodic Table from CK-12 Chemistry for High School
Explore the key groups of the periodic table, including Alkali Metals (Group 1), Alkaline Earth Metals (Group 2), Halogens (Group 7), Noble Gases (Group 8), and Transition Metals. This summary highlights their reactivity, electron configurations, and historical development by Dmitri Mendeleev.
Notes describing trends on the periodic table
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE Knowunity AI. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user