Carboxylic acids are everywhere around you - from the vinegar... Show more
Sign up to see the contentIt's free!
Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Subjects
3
0
sheila
12/5/2025
Chemistry
Chapter 26.3 - Carboxylic Acids
154
•
Dec 5, 2025
•
Carboxylic acids are everywhere around you - from the vinegar... Show more
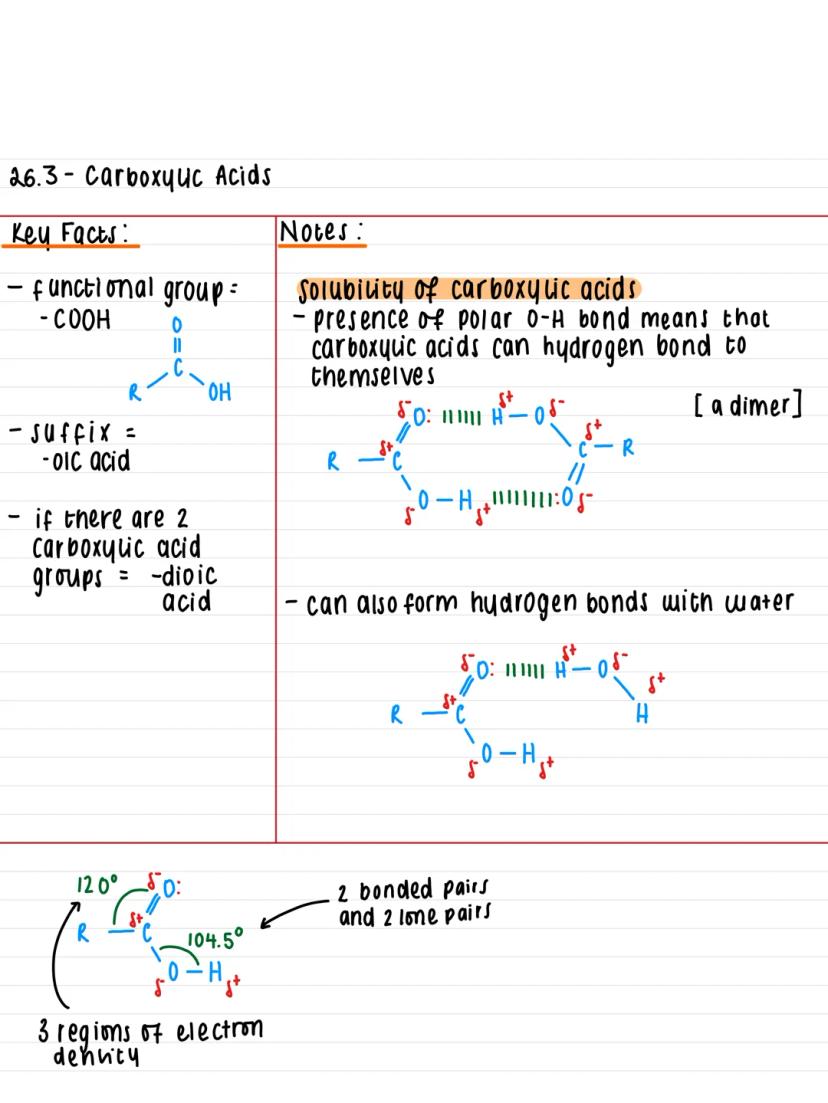

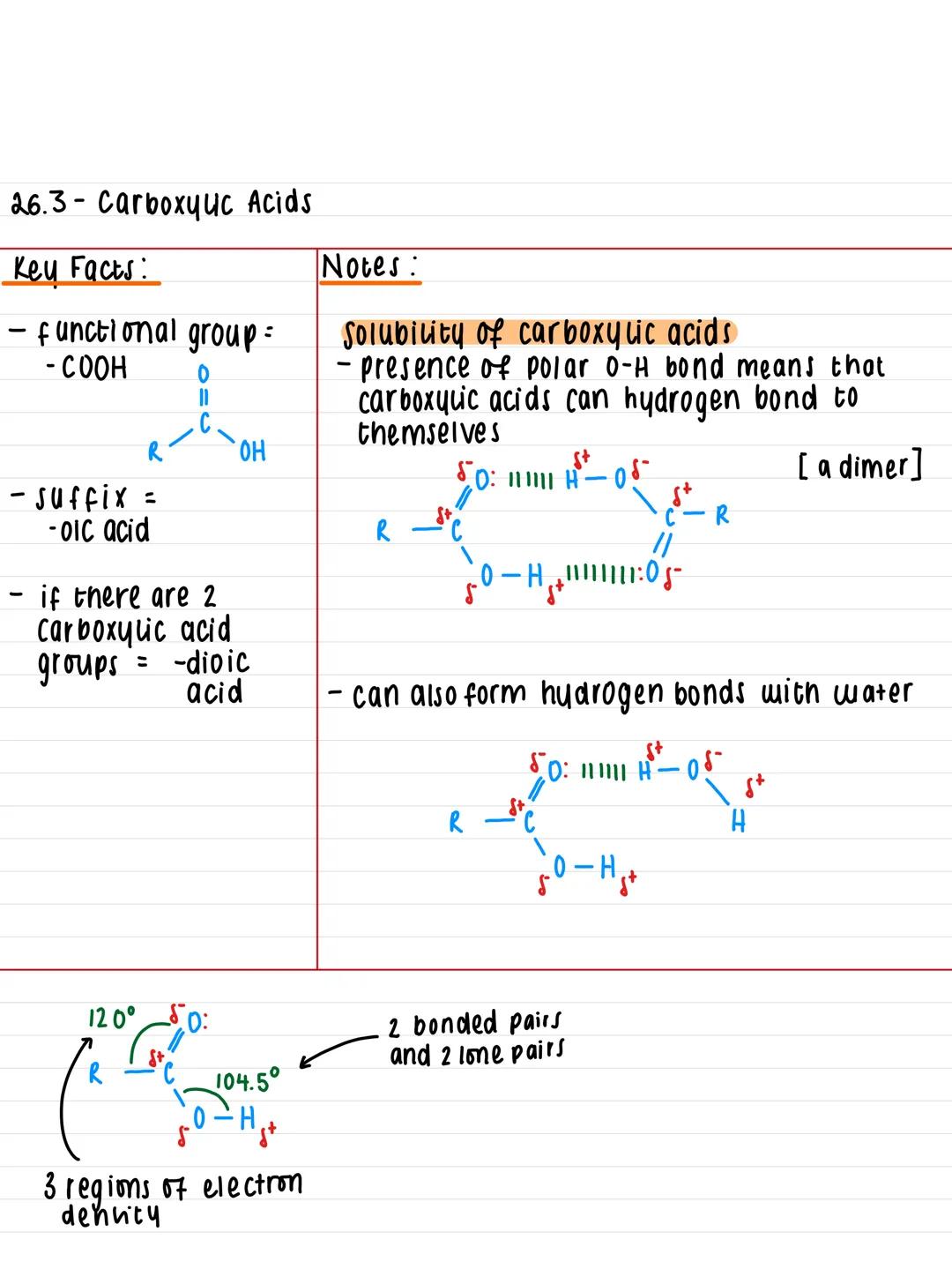
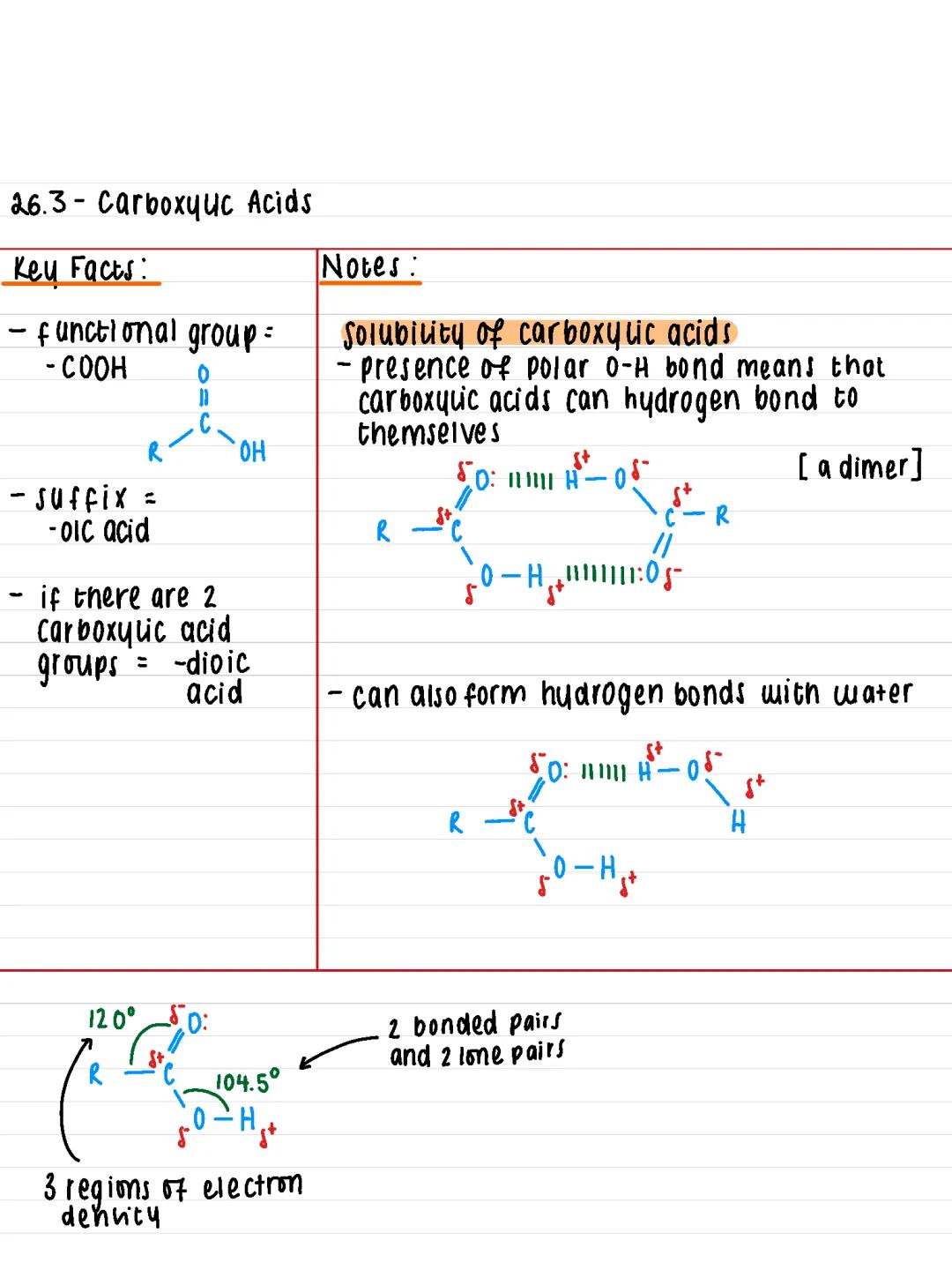
The functional group that defines carboxylic acids is -COOH, which gives them their distinctive properties. When naming these compounds, you simply add the suffix -oic acid to the end (like ethanoic acid for vinegar).
If a molecule has two carboxylic acid groups, you use -dioic acid instead. Think of it as doubling up on the acidity! The general structure shows a carbon atom bonded to both a hydroxyl group and a carbonyl group .
The geometry around the carbon is particularly important - you'll find three regions of electron density creating bond angles of 120°, whilst the oxygen in the -OH group has two bonded pairs and two lone pairs, giving it a 104.5° angle.
Key Insight: The -COOH group combines two familiar functional groups (carbonyl and hydroxyl), which explains why carboxylic acids are so reactive and versatile.
Hydrogen bonding is the secret behind carboxylic acids' unique physical properties. The polar O-H bond allows these molecules to form strong hydrogen bonds with each other, creating dimers (pairs of molecules stuck together).
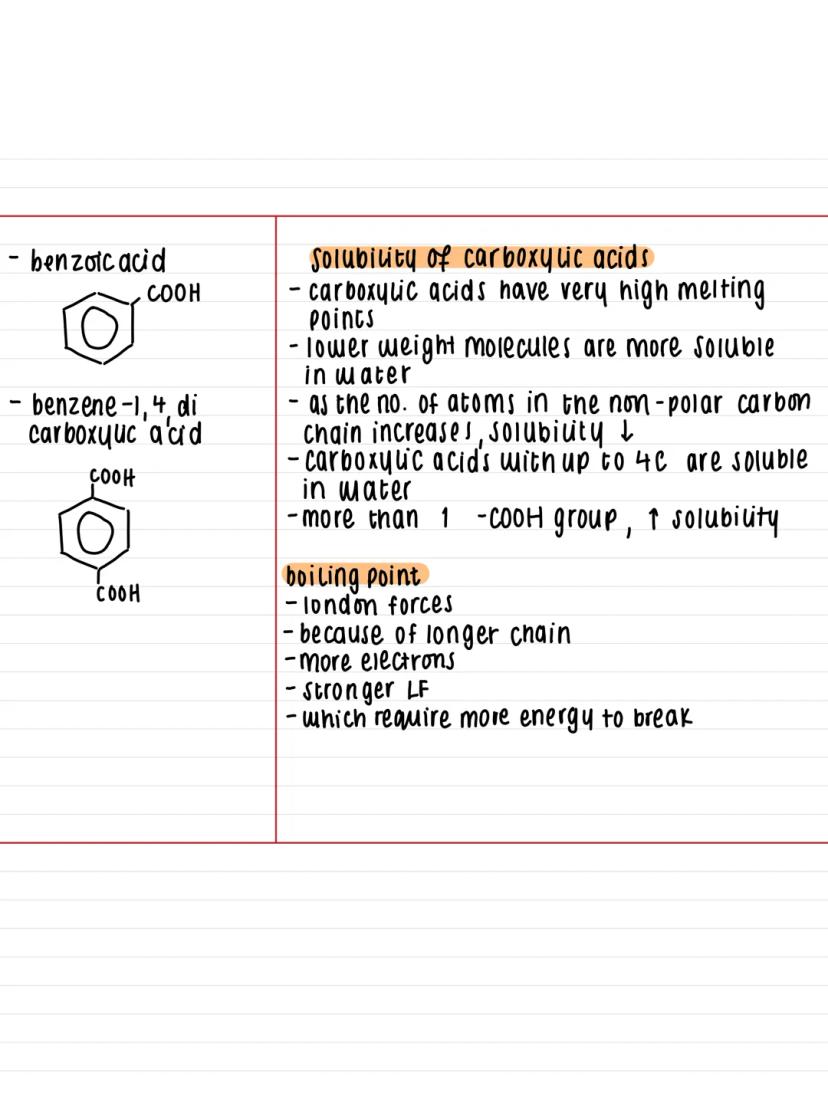
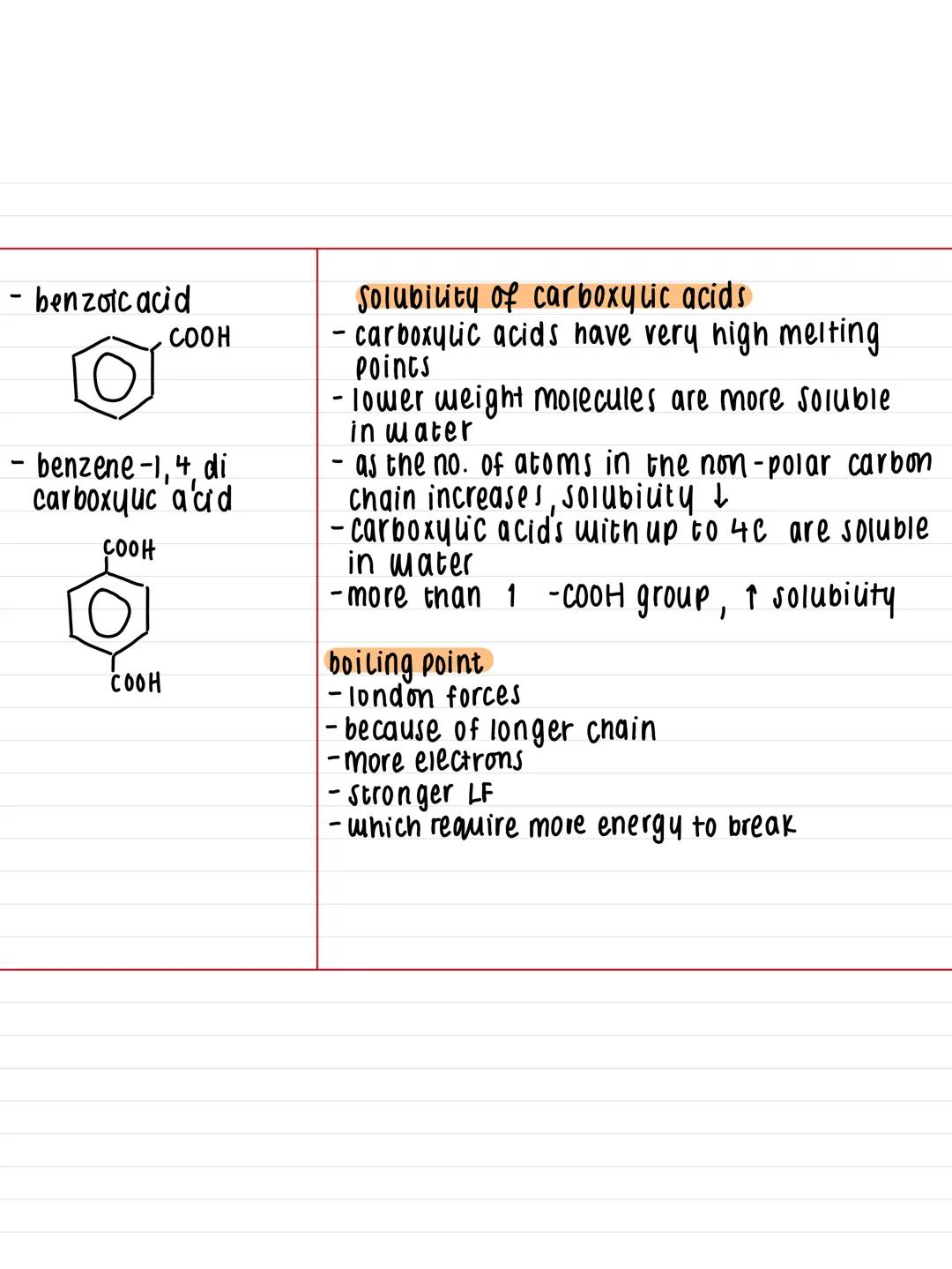
This hydrogen bonding also means carboxylic acids can bond effectively with water molecules. Carboxylic acids with up to 4 carbon atoms dissolve completely in water, but solubility drops dramatically as the carbon chain gets longer.
Here's the pattern you need to remember: longer carbon chains mean stronger London forces due to more electrons. This increases boiling points but decreases water solubility because the non-polar carbon chain dominates. However, if you've got more than one -COOH group, solubility increases again.
Exam Tip: Remember that melting and boiling points are unusually high for carboxylic acids due to that hydrogen bonding - this often comes up in exam questions!
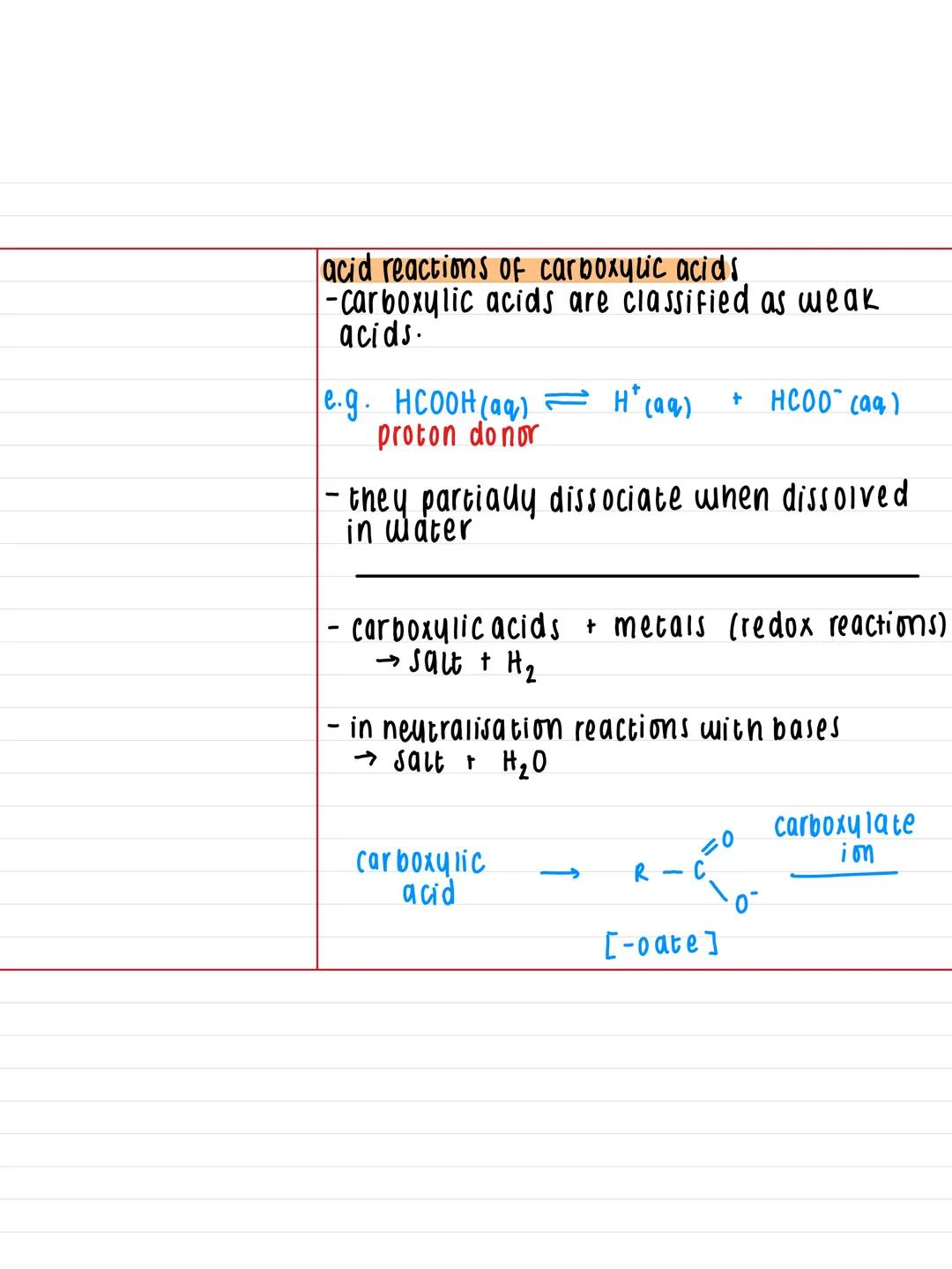
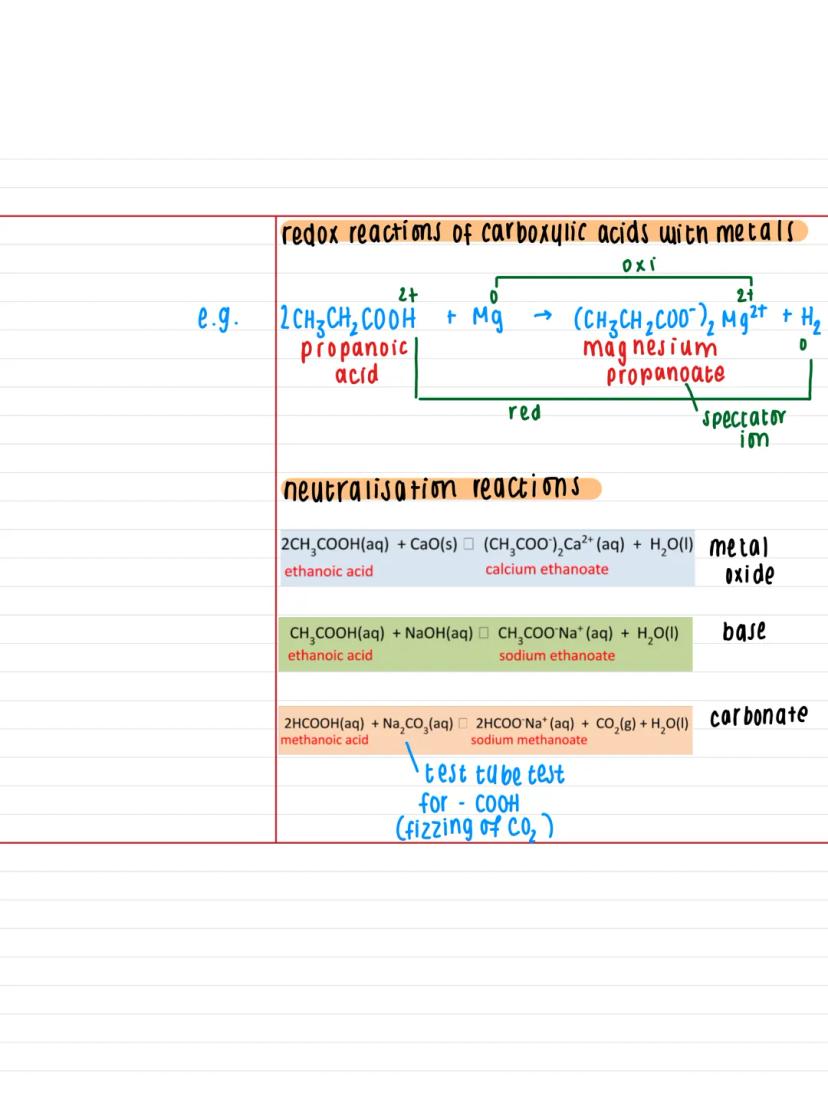
Carboxylic acids are weak acids, which means they only partially dissociate in water. For example, methanoic acid (HCOOH) splits into H⁺ and HCOO⁻ ions, but not completely - that's why we use the equilibrium arrow.
Since they're acids, they undergo typical acid reactions. With metals, you get a salt plus hydrogen gas (a redox reaction). With bases, you get neutralisation reactions producing a salt plus water.
The naming pattern for the salts is straightforward - just change the '-oic acid' ending to '-oate'. So ethanoic acid becomes ethanoate, propanoic acid becomes propanoate, and so on.
Memory Aid: Think of carboxylic acids as "polite acids" - they donate protons, but not too aggressively, unlike strong acids like hydrochloric acid.
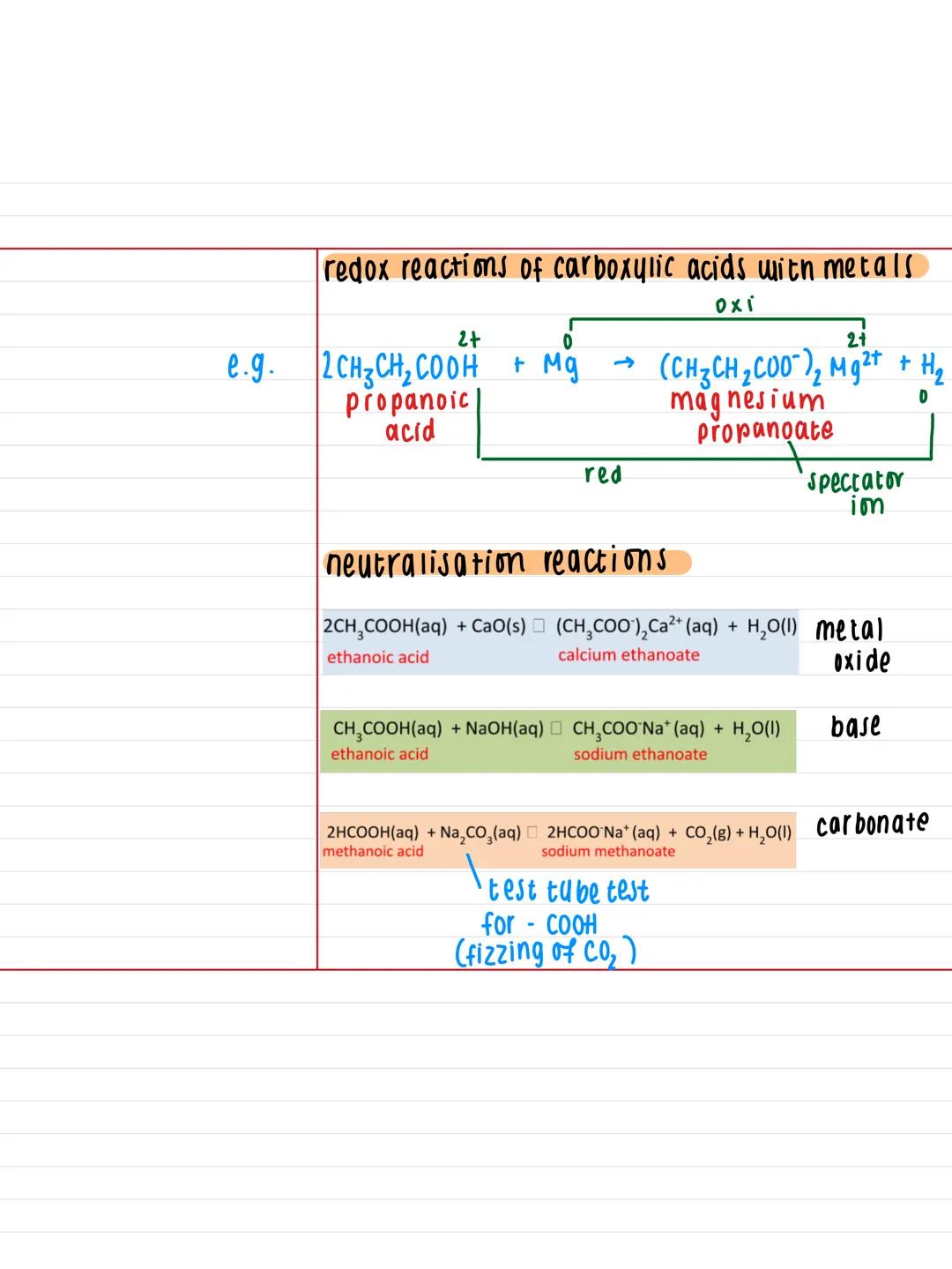
When carboxylic acids react with metals like magnesium, you get a classic redox reaction. For instance, propanoic acid + magnesium produces magnesium propanoate and hydrogen gas. The metal gets oxidised (loses electrons) whilst the hydrogen ions get reduced.
Neutralisation reactions follow predictable patterns. With metal oxides like calcium oxide, you get the metal carboxylate plus water. With hydroxides like sodium hydroxide, same result - salt plus water.
The test tube test for carboxylic acids uses sodium carbonate. If you add Na₂CO₃ to a carboxylic acid, you'll see fizzing as CO₂ gas is produced. This fizzing is your definitive test for the presence of a -COOH group.
Practical Note: That fizzing test with sodium carbonate is dead useful for identifying unknown compounds in your practicals - if it fizzes, you've likely got a carboxylic acid!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
Quotes from every main character
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
Carboxylic acids are everywhere around you - from the vinegar in your chips to the citric acid in your fizzy drinks. Understanding their structure and reactions is crucial for your A-level chemistry success, and once you grasp the basics, you'll... Show more

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
The functional group that defines carboxylic acids is -COOH, which gives them their distinctive properties. When naming these compounds, you simply add the suffix -oic acid to the end (like ethanoic acid for vinegar).
If a molecule has two carboxylic acid groups, you use -dioic acid instead. Think of it as doubling up on the acidity! The general structure shows a carbon atom bonded to both a hydroxyl group and a carbonyl group .
The geometry around the carbon is particularly important - you'll find three regions of electron density creating bond angles of 120°, whilst the oxygen in the -OH group has two bonded pairs and two lone pairs, giving it a 104.5° angle.
Key Insight: The -COOH group combines two familiar functional groups (carbonyl and hydroxyl), which explains why carboxylic acids are so reactive and versatile.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Hydrogen bonding is the secret behind carboxylic acids' unique physical properties. The polar O-H bond allows these molecules to form strong hydrogen bonds with each other, creating dimers (pairs of molecules stuck together).
This hydrogen bonding also means carboxylic acids can bond effectively with water molecules. Carboxylic acids with up to 4 carbon atoms dissolve completely in water, but solubility drops dramatically as the carbon chain gets longer.
Here's the pattern you need to remember: longer carbon chains mean stronger London forces due to more electrons. This increases boiling points but decreases water solubility because the non-polar carbon chain dominates. However, if you've got more than one -COOH group, solubility increases again.
Exam Tip: Remember that melting and boiling points are unusually high for carboxylic acids due to that hydrogen bonding - this often comes up in exam questions!

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
Carboxylic acids are weak acids, which means they only partially dissociate in water. For example, methanoic acid (HCOOH) splits into H⁺ and HCOO⁻ ions, but not completely - that's why we use the equilibrium arrow.
Since they're acids, they undergo typical acid reactions. With metals, you get a salt plus hydrogen gas (a redox reaction). With bases, you get neutralisation reactions producing a salt plus water.
The naming pattern for the salts is straightforward - just change the '-oic acid' ending to '-oate'. So ethanoic acid becomes ethanoate, propanoic acid becomes propanoate, and so on.
Memory Aid: Think of carboxylic acids as "polite acids" - they donate protons, but not too aggressively, unlike strong acids like hydrochloric acid.

Access to all documents
Improve your grades
Join milions of students
By signing up you accept Terms of Service and Privacy Policy
When carboxylic acids react with metals like magnesium, you get a classic redox reaction. For instance, propanoic acid + magnesium produces magnesium propanoate and hydrogen gas. The metal gets oxidised (loses electrons) whilst the hydrogen ions get reduced.
Neutralisation reactions follow predictable patterns. With metal oxides like calcium oxide, you get the metal carboxylate plus water. With hydroxides like sodium hydroxide, same result - salt plus water.
The test tube test for carboxylic acids uses sodium carbonate. If you add Na₂CO₃ to a carboxylic acid, you'll see fizzing as CO₂ gas is produced. This fizzing is your definitive test for the presence of a -COOH group.
Practical Note: That fizzing test with sodium carbonate is dead useful for identifying unknown compounds in your practicals - if it fizzes, you've likely got a carboxylic acid!
Our AI companion is specifically built for the needs of students. Based on the millions of content pieces we have on the platform we can provide truly meaningful and relevant answers to students. But its not only about answers, the companion is even more about guiding students through their daily learning challenges, with personalised study plans, quizzes or content pieces in the chat and 100% personalisation based on the students skills and developments.
You can download the app in the Google Play Store and in the Apple App Store.
That's right! Enjoy free access to study content, connect with fellow students, and get instant help – all at your fingertips.
3
Smart Tools NEW
Transform this note into: ✓ 50+ Practice Questions ✓ Interactive Flashcards ✓ Full Mock Exam ✓ Essay Outlines
Explore the fundamentals of buffer solutions, including preparation methods, the role of weak acids and their conjugate bases, and calculations for pH using the Henderson-Hasselbalch equation. This summary covers key concepts such as acid dissociation constant (Ka), buffer capacity, and the equilibrium shifts in response to added acids or alkalis. Ideal for students studying chemistry and seeking to understand the principles behind buffer systems.
Explore the fundamentals of acids and alkalis, including the pH scale, properties of common acids and bases, and the process of salt formation through neutralization reactions. This summary covers key concepts such as strong and weak acids, polyatomic ions, and practical applications in chemistry. Ideal for students preparing for exams or seeking to deepen their understanding of acid-base chemistry.
This tackles the general properties of acids and bases, their examples and pH concept.
Quotes from every main character
App Store
Google Play
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user
The app is very easy to use and well designed. I have found everything I was looking for so far and have been able to learn a lot from the presentations! I will definitely use the app for a class assignment! And of course it also helps a lot as an inspiration.
Stefan S
iOS user
This app is really great. There are so many study notes and help [...]. My problem subject is French, for example, and the app has so many options for help. Thanks to this app, I have improved my French. I would recommend it to anyone.
Samantha Klich
Android user
Wow, I am really amazed. I just tried the app because I've seen it advertised many times and was absolutely stunned. This app is THE HELP you want for school and above all, it offers so many things, such as workouts and fact sheets, which have been VERY helpful to me personally.
Anna
iOS user
I think it’s very much worth it and you’ll end up using it a lot once you get the hang of it and even after looking at others notes you can still ask your Artificial intelligence buddy the question and ask to simplify it if you still don’t get it!!! In the end I think it’s worth it 😊👍 ⚠️Also DID I MENTION ITS FREEE YOU DON’T HAVE TO PAY FOR ANYTHING AND STILL GET YOUR GRADES IN PERFECTLY❗️❗️⚠️
Thomas R
iOS user
Knowunity is the BEST app I’ve used in a minute. This is not an ai review or anything this is genuinely coming from a 7th grade student (I know 2011 im young) but dude this app is a 10/10 i have maintained a 3.8 gpa and have plenty of time for gaming. I love it and my mom is just happy I got good grades
Brad T
Android user
Not only did it help me find the answer but it also showed me alternative ways to solve it. I was horrible in math and science but now I have an a in both subjects. Thanks for the help🤍🤍
David K
iOS user
The app's just great! All I have to do is enter the topic in the search bar and I get the response real fast. I don't have to watch 10 YouTube videos to understand something, so I'm saving my time. Highly recommended!
Sudenaz Ocak
Android user
In school I was really bad at maths but thanks to the app, I am doing better now. I am so grateful that you made the app.
Greenlight Bonnie
Android user
I found this app a couple years ago and it has only gotten better since then. I really love it because it can help with written questions and photo questions. Also, it can find study guides that other people have made as well as flashcard sets and practice tests. The free version is also amazing for students who might not be able to afford it. Would 100% recommend
Aubrey
iOS user
Best app if you're in Highschool or Junior high. I have been using this app for 2 school years and it's the best, it's good if you don't have anyone to help you with school work.😋🩷🎀
Marco B
iOS user
THE QUIZES AND FLASHCARDS ARE SO USEFUL AND I LOVE THE SCHOOLGPT. IT ALSO IS LITREALLY LIKE CHATGPT BUT SMARTER!! HELPED ME WITH MY MASCARA PROBLEMS TOO!! AS WELL AS MY REAL SUBJECTS ! DUHHH 😍😁😲🤑💗✨🎀😮
Elisha
iOS user
This app is phenomenal down to the correct info and the various topics you can study! I greatly recommend it for people who struggle with procrastination and those who need homework help. It has been perfectly accurate for world 1 history as far as I’ve seen! Geometry too!
Paul T
iOS user